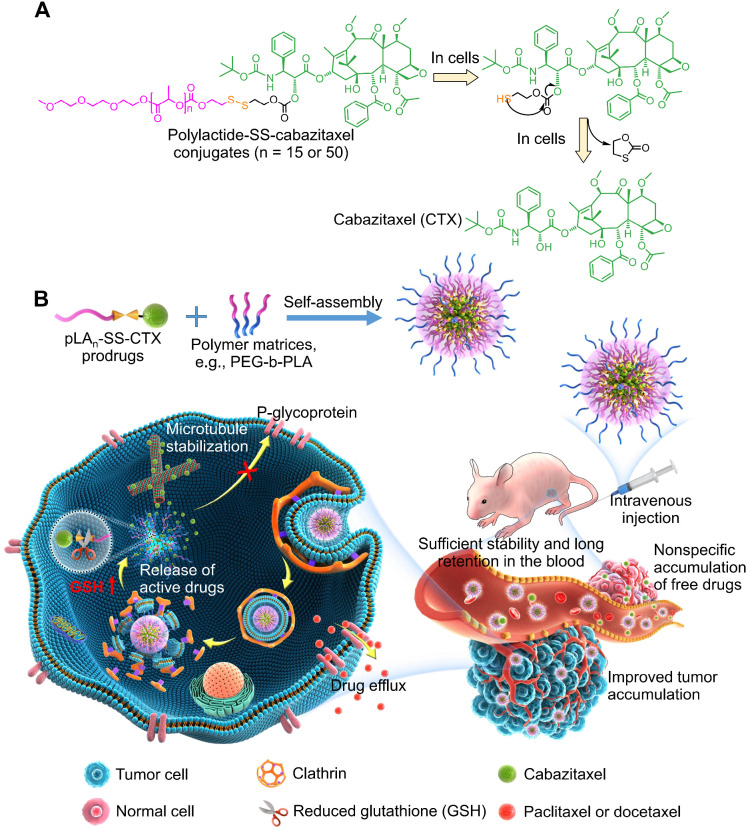
Figure 1.
A) Chemical structure of polylactide-SS-cabazitaxel conjugates (termed pLAn-SS-CTX) and drug activation in cells in response to reduced glutathione (GSH). B) Schematic illustration of self-assembly of prodrugs with amphiphilic copolymers for tumor-specific and on-demand delivery of hydrophobic cabazitaxel agent and systemic treatment of taxane-resistant cancer. Following intravenous injection, the nanomedicines are sufficiently stable to prevent premature release of toxic drugs and to reduce systemic toxicity. Once accumulated at tumor sites via passive targeting, nanoparticles are internalized by cancer cells via clathrin-mediated endocytosis. Finally, the particles release pharmacologically active cabazitaxel through the cleavage of the disulfide bond in cells, which spontaneously induces cell apoptosis.