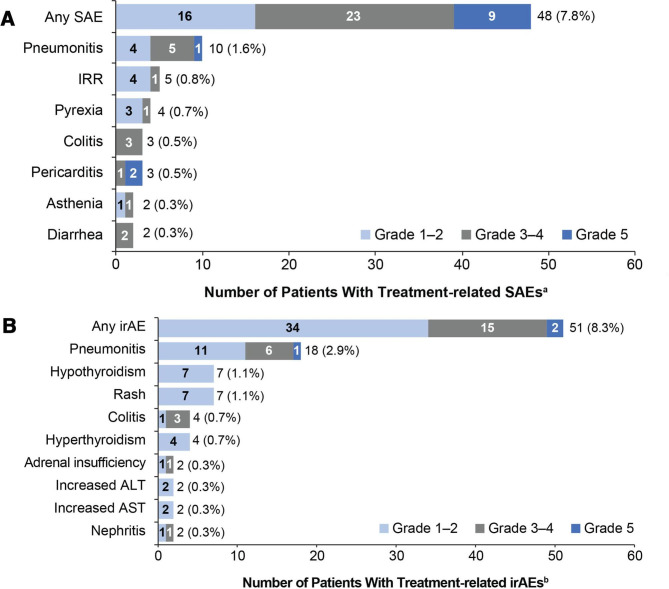
Figure 2.
Primary endpoint: most common treatment-related SAEs and irAEs (in ≥2 patients) by CTCAE grade. (A) Treatment-related SAEs and (B) treatment-related irAEs. aAdverse events were defined as serious if they were fatal, were life threatening, required hospitalization, resulted in disability, or resulted in a congenital birth defect in an infant born to a mother exposed to study drug. birAEs were defined as any adverse event of special interest requiring corticosteroid treatment within 30 days of onset. ALT, alanine aminotransferase; AST, aspartate aminotransferase; CTCAE, Common Terminology Criteria for Adverse Events; irAEs, immune-related adverse events; IRR, infusion-related reaction; SAE, serious adverse event.