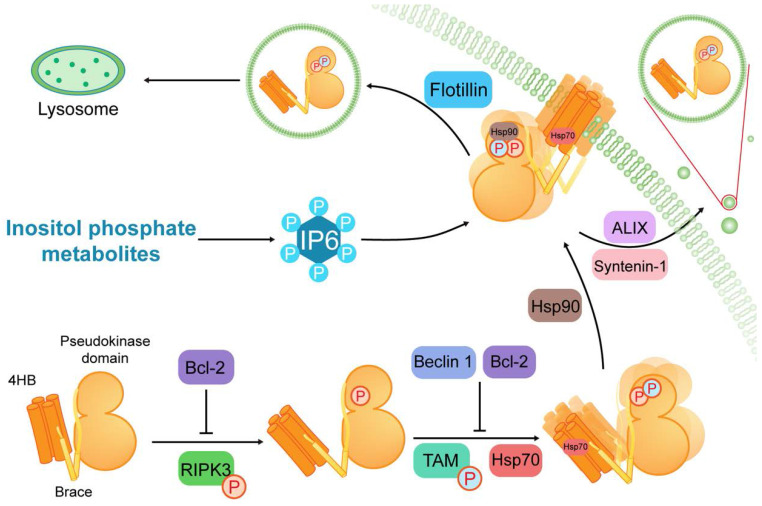
Figure 1.
Structure of MLKL and its regulation in necroptosis. MLKL contains a C-terminal pseudokinase domain, a two-helix brace and an N-terminal four-helix bundle (4HB). After MLKL is phosphorylated by RIPK3, TAM (Tyro3, Axl, and Mer) kinase phosphorylates MLKL at the pseudokinase domain to initiate oligomerization of MLKL. Then MLKL is conjugated to heat shock protein 90 (Hsp90) and Hsp70 to achieve membrane translocation. Furthermore, inositol phosphate metabolites are required for the IP6 production. And IP4, IP5 and IP6 serve as critical binders of MLKL in necroptosis. During the course of MLKL activation, Beclin 1 and B-cell lymphoma 2 (Bcl-2) play negative regulatory roles in the conformation and oligomerization of MLKL. Additionally, flotillin-mediated endocytosis and ALIX-syntenin-1-mediated exocytosis can preclude contact between MLKL and the plasma membrane to suppresses necroptosis.