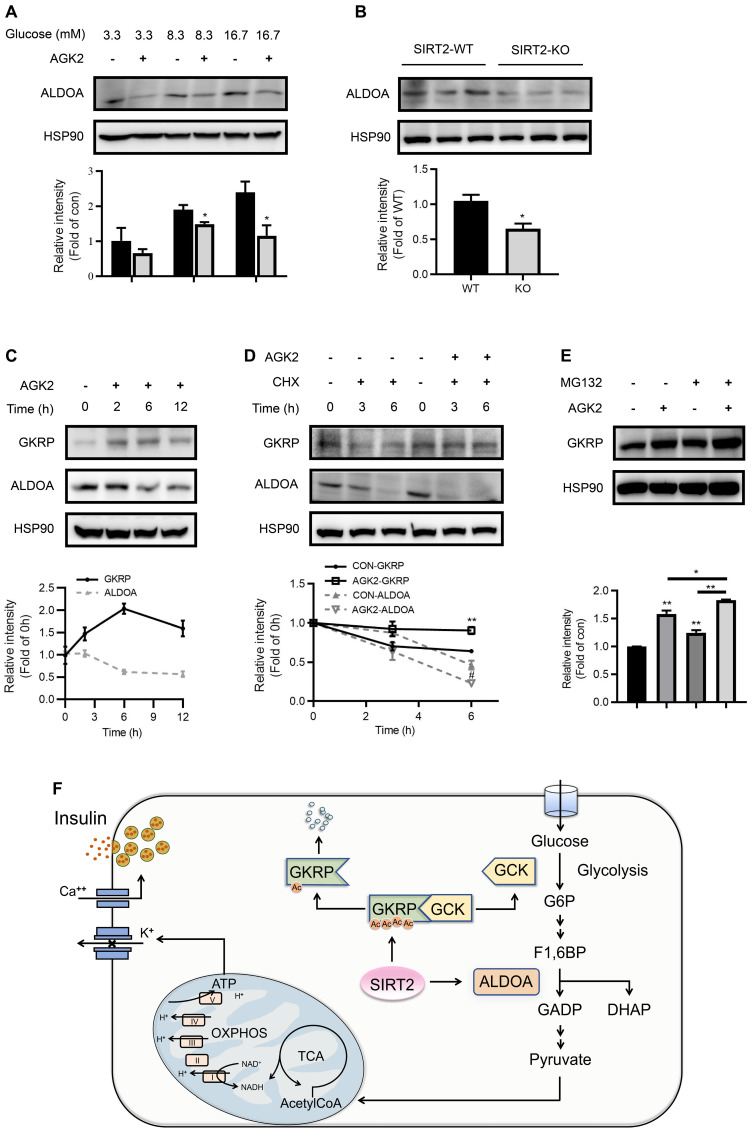
Figure 7.
SIRT2 inhibition promotes GKRP protein stability and ALDOA protein degradation. (A) ALDOA protein level in INS-1 cells treated with 3 μM AGK2 in the presence of 3.3, 8.3 or 16.7 mM glucose. (B) Protein expression of ALDOA in the islets isolated from SIRT2-WT and SIRT2-KO rats. (C) GKRP and ALDOA protein expressions in INS-1 cells incubated with 3 μM AGK2 for the indicated time points. (D) GKRP and ALDOA protein expressions in INS-1 cells exposed to 3 μM AGK2 and 10 μg/ml cycloheximide (CHX) for 3 and 6 h. (E) GKRP protein expression in INS-1 cells treated with or without 3 μM AGK2 and 10 μM MG132 for 6 h. Signal intensity was quantified by image J software for statistical comparison. (F) The schematic illustration summarizes the role of SIRT2 in glucose-stimulated insulin secretion. GCK acts as a glucose sensor in the β-cells by converting glucose to G6P, which eventually yields ATP through glycolysis, TCA cycle, and oxidative phosphorylation for triggering insulin secretion. GKRP binds and inactivates GCK. SIRT2 inhibits ALDOA protein degradation and decreases GKRP protein stability by decreasing its acetylation level, thereby disrupting the GKRP-GCK complex and promoting glycolytic flux and insulin secretion. Data are expressed as means ± SEM. *P< 0.05, **P< 0.01 vs control group. #P< 0.05 vs CON-ALDOA group.