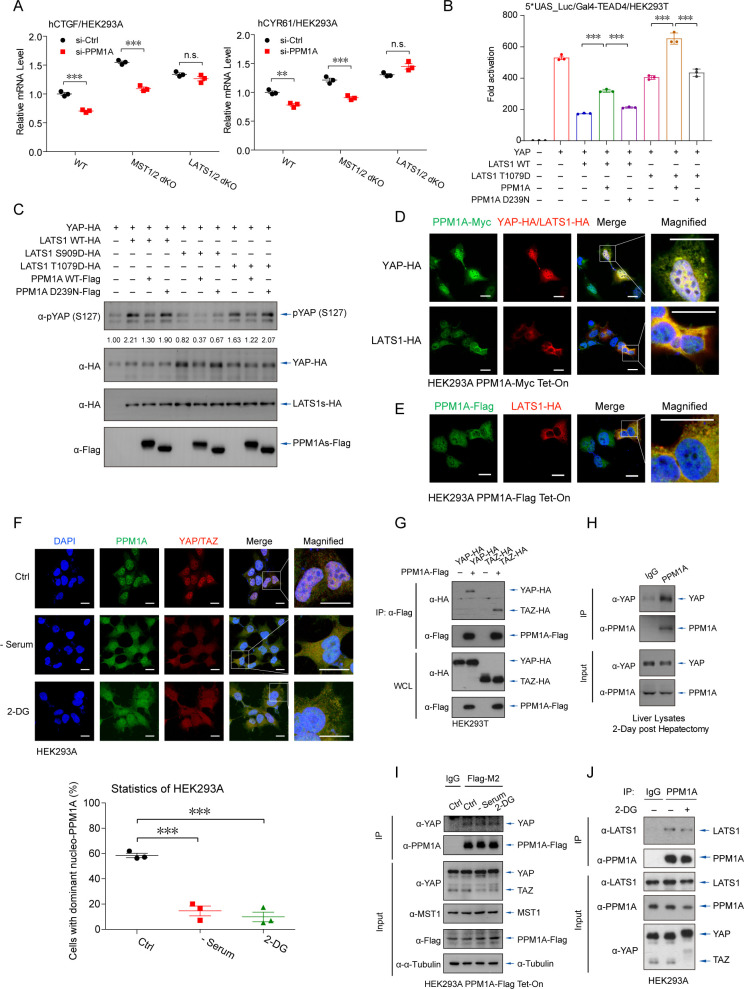
Fig 3. PPM1A translocates and associates with YAP/TAZ and LATS kinases.
(A) Upon PPM1A depletion, qRT-PCR assays detected the significantly lower mRNA levels of YAP/TAZ target genes in WT or MST1/2 dKO cells, but not in LATS1/2 dKO cells. (B, C) The transcription potency of YAP that was inhibited by LATS1 T1079D, a LATS1 phosphomimetic, was restored by PPM1A cotransfection (B). LATS1 T1079D-stimulated phospho-YAP (S127) was eliminated by PPM1A, similar to those phospho-YAP (S127) induced by WT LATS1 (C). LATS1 S909D failed to phosphorylate and inactivate YAP. (D, E), Immunofluorescence imaging detected an overlap of cellular localization between PPM1A and YAP, occurred in the nucleus and in the cytoplasm (D). In the presence of LATS1, PPM1A translocated from the nucleus to the cytoplasm, where it overlapped with LATS1 (D and E). (F) In response to nutrient deficiency that activated Hippo signaling, endogenous PPM1A was partially exported to the cytoplasm, where it colocalized with YAP. Percentage of cells with above 50% PPM1A in the nucleus was counted (bottom panels). (G–I) The complex between PPM1A and YAP/TAZ was detected by coimmunoprecipitation assays, when these proteins were coexpressed (G), the endogenous YAP and PPM1A in liver lysates upon 2 dph (H), or between endogenous YAP and stably expressed PPM1A (I). (J) The endogenous complex of PPM1A-LATS1 was also visible in HEK293 cells, as revealed by coimmunoprecipitation assays using an anti-PPM1A antibody. Unprocessed images of blots are shown in S1 Raw Images. Statistics source data are provided in S1 Data. dKO, double knockout; dph, days post hepatectomy; LATS, large tumor suppressor kinase; PPM1A, protein phosphatase magnesium-dependent 1A; qRT-PCR, real-time quantitative PCR; TAZ, transcriptional coactivator with PDZ-binding motif; WT, wild-type; YAP, Yes-associated protein.