Fig. 8.
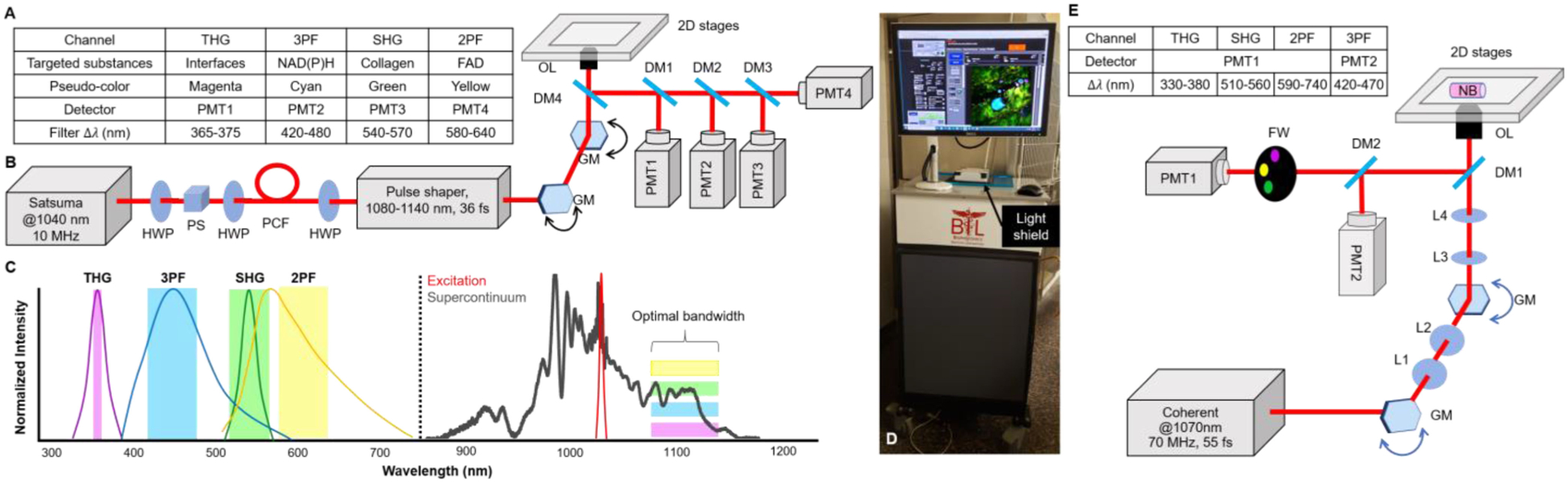
System design comparison between a bench-top SLAM microscope and an intraoperative NLOI system. (A) Table summarizing the color code, corresponding detector, and filtered spectral bandwidth of each individual channel in the SLAM microscope. (B) Optical setup of the SLAM microscope. The system laser source operated at 1040 nm with a 10 MHz repetition rate and was first sent through the photonic crystal fiber (PCF) to generate a supercontinuum (full spectrum shown in (C)). A pulse shaper was programmed to choose the optimal excitation window (1080–1140 nm) and compensate the dispersion for a nearly transform-limited output pulse duration of around 36 fs. Lenses between the galvanometer mirror (GM) scanners and between GM and the objective lens (OL) were omitted for simplification. Different dichroic mirrors (DMs) and optical filters were used before photomultipliers (PMTs) as specified in the table in (A). (C) Normalized spectra and spectral windows for the four different channels listed in the table in (A), excited by the optimal window of the supercontinuum (dark grey) source. (D) Photograph of the portable intraoperative NLOI system with a light shield covering the specimen. (E) Optical setup of the intraoperative NLOI system with a table summarizing differences in the four-channel acquisition. The femtosecond laser centered at 1070 nm excited all four channels simultaneously. PMT1 collects the signals from THG, SHG and 2PF sequentially via filter wheel (FW) switching, while PMT2 simultaneously detects the weaker 3PF signals. The light shield is not drawn in the schematic. HWP, half wave plate; L, lens; NB, needle biopsy specimen; PS, polarization beam splitter; PM, parabolic mirror.
