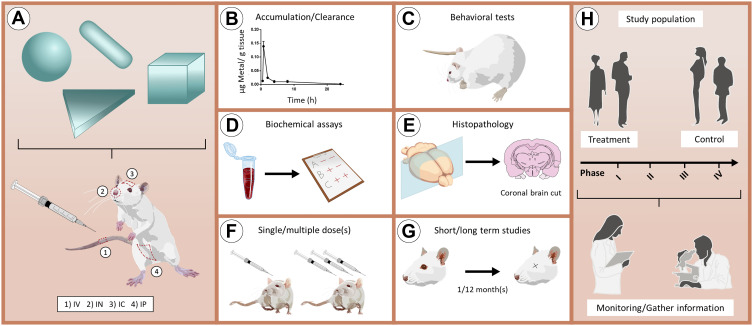
Figure 6.
Ideal methodology to assess the toxicity of GNPs/SNPs for applications in brain-related disorders. (A) Schematic representation of in vivo administration of nanoparticles with different shapes through the main routes of administration. (B–G) Main in vivo experiments that can be performed to obtain a complete toxicological profile of nanoparticles. (H) Representation of a clinical trial that includes treatment and control groups. During the different phases of the clinical trials (I–IV), it is relevant to monitor individuals to gather essential information.
Abbreviations: IV, intravenous; IN, intranasal; IC, intracranial; IP, intraperitoneal.