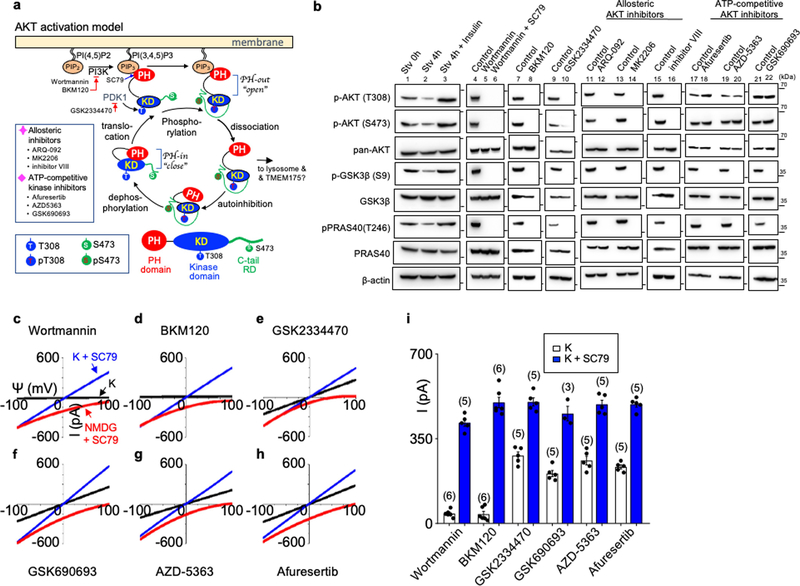
Extended Data Fig. 5 |. AKT activates TMEM175 in a catalytic activity-independent manner.
a, Schematic of the canonical AKT activation, with inhibitors used to target each step indicated, modified from previous publications23,80. A domain structure of AKT is in the bottom. AKT has a PH domain (PH), a kinase domain (KD) and a C-terminal tail regulatory domain (RD). b, Phosphorylation status of AKT and its two canonical targets GSK3β and PRAS40 following treatments similar to those used in the patch-clamp recording of TMEM175. Lysates from human TMEM175-transfected cells were probed with antibodies as indicated. Treatment conditions were: starvation (0 h, 4 h in HBSS) without (lanes 1 and 2) or with insulin (lane 3, 100 nM, 4 h) treatment afterwards, wortmannin (20 μM, 2 h) with and without SC79 (10 μM, 30 min) treatment afterwards (lanes 5 and 6), BKM120 (10 μM, 3 h; lane 8), GSK2334470 (20 μM, 3 h; lane 10), ARQ-092 (20 μM, 3 h; lane 12), MK2206 (20 μM, 3 h; lane 14), inhibitor VIII (10 μM, 3 h; lane 16), afuresertib (20 μM, 3 h; lane 18), AZD-5363 (20 μM, 3 h; lane 20), GSK690693 (10 μM, 4 h; lane 22). For gel source data, see Supplementary Fig. 1. c–i, Lysosomal IK was recorded from TMEM175-transfected HEK293T cells pretreated with wortmannin (c), BKM120 (d), GSK2334470 (e), GSK690693 (f), AZD-5363 (g) or afluresertib (h) as in b before lysosomal dissection for recording. Averaged IK sizes (at 100 mV) are shown in i. Data are mean ± s.e.m. Numbers of recordings are in parentheses. Our finding that SC79 fully activates TMEM175 in starved lysosomes was surprising but is also consistent with the idea that channel activation is independent of the kinase activity of AKT. Upon receptor activation by growth factors, PI3 kinases (PI3Ks) increase the concentration of PIP3 from PtdIns(4,5) P2 (PIP2)18. SC79 and the endogenous AKT activator PIP3 both bind to the PH domain of AKTs to induce large conformational changes that lead to the release of the PH domain from the catalytic domain and to an open state of the kinase19, which renders AKT accessible to phosphorylation at a key residue in the kinase domain (T308 in human AKT1) by PKD1 and another residue (S473) in the C-terminal regulatory domain by kinases such as mTORC2. Phosphorylation of T308 and S473 is required for the efficient activation of AKT kinase activity to phosphorylate downstream targets such as GSK3β and PRAS4018 (a). In nutrient-replete cells, TMEM175-associated AKT is at least partially phosphorylated at the two sites (Extended Data Fig. 4f). We used a panel of AKT inhibitors and mutants to probe the involvement of each of the steps in AKT function in TMEM175 activation. In starved cells and wortmannin-treated ones, AKT kinase activity is minimal because of the lack of phosphorylation of T308 on AKT by PKD1. Indeed, the activation of AKT kinase activity by SC79 on AKT isolated from starved cells requires the addition of PKD1 and ATP. Similarly, SC79 does not induce substantial AKT kinase activity (assayed with target phosphorylation) in wortmannin-treated cells19 (b). However, SC79 maximally activated TMEM175 in lysosomes isolated from starved cells (Fig. 2d, h) and in those from wortmannin-treated cells (c, i), without PKD1 or ATP in the bath, supporting that the SC79-induced channel activation does not require the kinase activity of AKT. Reduction of PIP3 by starvation and by inhibition of PI3K with wortmannin or BKM120 (a specific PI3K inhibitor)—treatments expected to keep AKT in a PH-in a closed state—both reduced the phosphorylation of AKT at T308 and S473 and that of the downstream AKT targets GSK3 (S9) and PRAS40 (T246) (b). The treatments also eliminated ITMEM175, which was restored by SC79 application (b–d, i). These data suggest that AKT activates channel after it switches from the PH-in closed state to the PH-out open state. To further test whether—after AKT switches to a PH-out open state—phosphorylation of T308 in AKT by PDK1 is required for ITMEM175, we inhibited PDK1 with GSK2334470. Although the inhibitor abolished the phosphorylation of AKT and its downstream targets, it had little effect on ITMEM175 (b, e, i). Catalytic and allosteric AKT inhibitors both block phosphorylation of downstream targets by AKT, but via different mechanisms18 (a). Unlike allosteric inhibitors, which probably keep AKT in a closed phosphorylation-resistant state and prevent the activation of TMEM175 (b, Fig. 2f), catalytic inhibitors such as those that compete with ATP binding in the activation loop are downstream of the phosphorylation of AKT T308 and S473 and inhibit AKT kinase activities in an open state. The ATP competitive inhibitors GSK690693, AZD-5363 and GSK2110183 (afuresertib) all inhibited AKT target phosphorylation (b), but—in contrast to the allosteric inhibitors (Fig. 2f)—had little effect on ITMEM175 (f–i). Together, the inhibitors targeting different steps of AKT activation suggest that conformational changes that lead to AKT in an open state, but not the downstream steps of the catalytic activities of AKT, are required for ITMEM175.