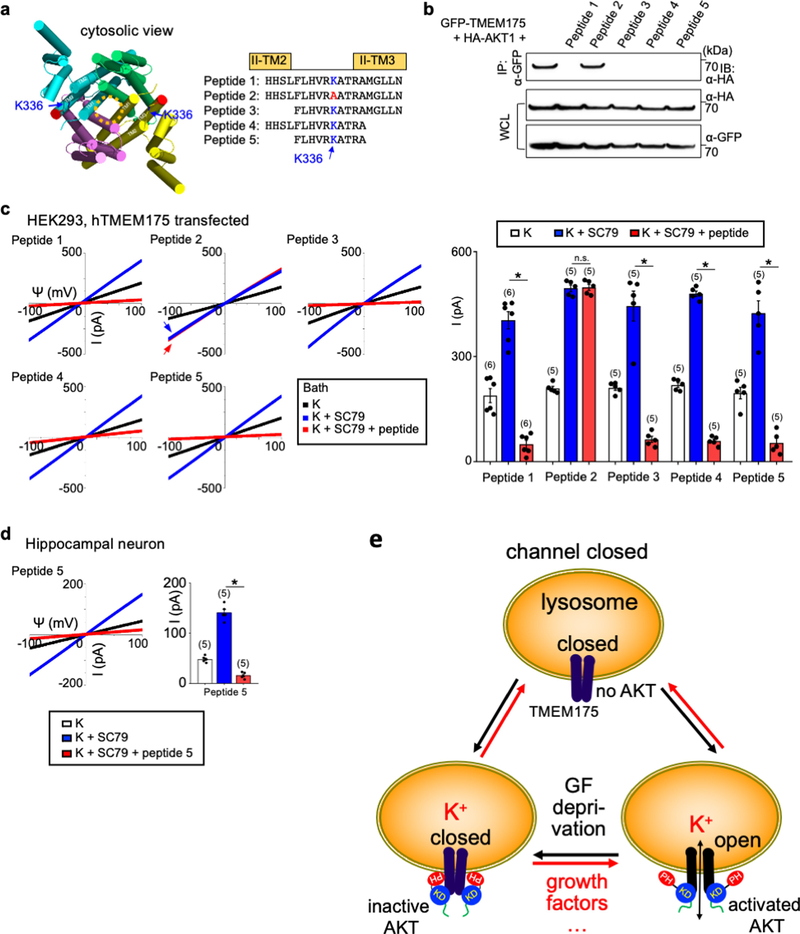
Extended Data Fig. 7 |. Association of AKT to TMEM175 is required to maintain ITMEM175 after the channel is activated.
a, b, Development of peptide inhibitors that dissociate AKT from TMEM175 in the lysoKGF complex. a, Sequences of synthesized human TMEM175 peptides around the K336 region that interacts with AKT. Peptide 2 has a K336A mutation. b, To test the ability of each peptide to dissociate AKT from TMEM175, cell lysates from HEK293T cells cotransfected with GFP-tagged human TMEM175 and HA-tagged human AKT1 were incubated with each peptide (10 μM) for 2 h before immunoprecipitation with anti-GFP to bring down TMEM175 and immunoblotting with anti-HA to detect the associated AKT1. Immunoprecipitation without peptide incubation (lane 1) was used as a control. Whole-cell lysate was also blotted with anti-HA and anti-GFP for input control. For gel source data, see Supplementary Fig. 1. The results show that when applied in cell lysates containing the TMEM175–AKT complex, peptides as short as 10 amino acids (peptide 5) in the K336 region were sufficient to dissociate AKT from the preassembled AKT–TMEM175 complex in the immunoprecipitation assay. As a control, K336A mutation (peptide 2) abolished the dissociation ability of the peptide. c, d, Lysosomal IK were recorded from TMEM175-transfected HEK293T cells (c) and mouse hippocampal neurons (d). After SC79 application to maximally activate lysoKGF, peptide inhibitor (10 μM) was added to the bath and currents were recorded for about 10 min until they reached steady states. The K336A mutant peptide 2 was used as a negative control. Averaged IK sizes (at 100 mV) are shown in the bar graphs. In c subpanel ‘peptide 2’, arrows are used to indicate curves that overlap and are not easily distinguished. Blue, from bath containing K+ and SC79 before peptide application; red, from bath containing K+ and SC79 after peptide application. Data are mean ± s.e.m. Numbers of recordings are in parentheses. *P ≤ 0.05. P values (unpaired two-tailed t-tests) (K+SC79 vs K+SC79 + peptide groups) are as follows. In c, P = 0.8447 for peptide 2, P < 0.0001 for the other treatments; in d, P = 0.0011 for peptide 5. The results show that after the channel was maximally activated by SC79, bath application of AKT-association peptide inhibitors—but not the one with the K336A point mutation—diminished both heterologously expressed ITMEM175 in HEK293T cells and the native currents in neurons. Given that the bath contained no phosphatase, the action of the peptides is unlikely through a dephosphorylation of the channel target but is rather through the dissociation of AKT from TMEM175—further supporting the idea that it is the conformational change of AKT, and not target phosphorylation, that activates the channel. e, A model for TMEM175 activation by AKT conformational changes. TMEM175 without AKT or associated with inactive AKT in its PH-in closed conformation is closed. The channel opens upon AKT conformational changes triggered by physiological activators such as extracellular growth factors, lipids, or synthetic small molecules such as SC79. The gating mechanism does not require AKT kinase activity. AKT mutations found in cancers that alter the interaction between the PH and kinase domains also alter TMEM175 channel property.