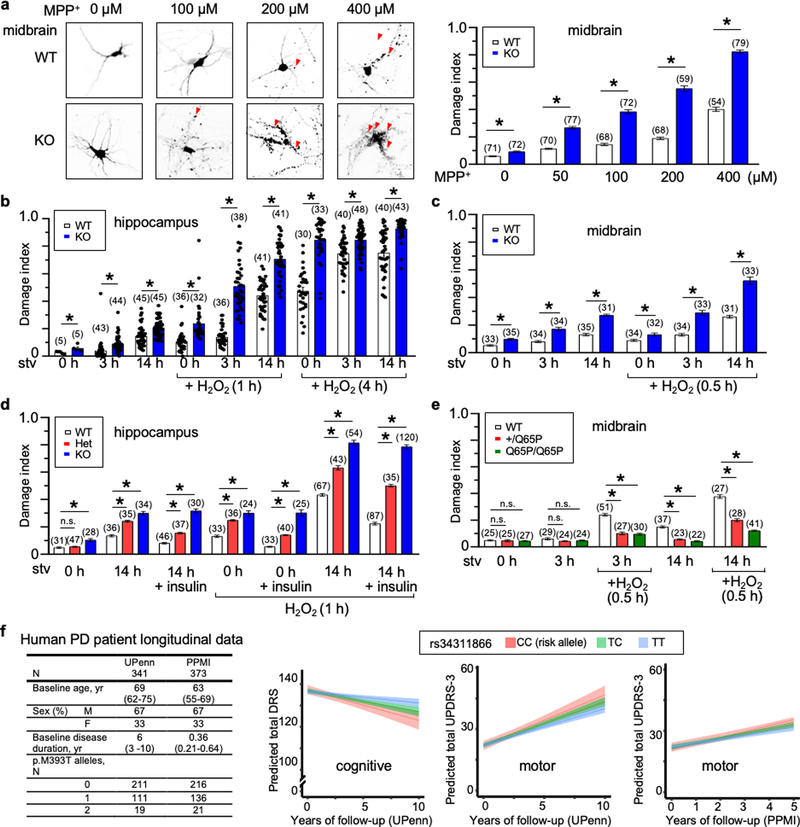
Extended Data Fig. 10 |. LysoKGF protects mouse neurons from stress-induced damage in a gene-dosage-dependent manner, and a loss-of-function variant in the human TMEM175 gene is nominally associated with increased rates of cognitive and motor function decline in patients with Parkinson’s disease.
a–e, Cultured mouse neurons were challenged with MPP+ toxin, B27 deprivation (in DMEM) and/or H2O2 treatment. Images were taken for damage analysis. Blebs and fragmentation in neuronal processes were counted as damage, as indicated by arrows in representative pictures in a. The damage index was calculated as the ratio of damaged area to the total neuronal area. a, Treatment of wild-type and TMEM175-knockout midbrain neurons with MPP+ added to culture medium for 12 h, with concentrations as indicated. Left, representative images of neurons with bleb damages indicated by arrows. Right, damage index. b, c, Hippocampal (b) or midbrain (c) neurons were starved in DMEM for duration as indicated. H2O2 (100 μM) treatment for the period indicated was carried out in DMEM after starvation. d, Effects of insulin. In the group of neurons treated with 14-h starvation and insulin, without H2O2, neurons were starved for 14 h in DMEM followed by incubation with insulin (100 nM in DMEM) for 3 h. In the groups treated with H2O2, neurons were starved in DMEM for 0 or 14 h, followed by a 3-h incubation with or without insulin (100 nM). During the last hour of incubation, H2O2 (100 μM) was added to the medium. e, Comparison between wild-type and TMEM175(Q65P)-carrying neurons with treatments similar to those in c. In the bar graphs, data are mean ± s.e.m.; numbers of neurons analysed are in parentheses. *P ≤ 0.05. P values (unpaired two-tailed t-tests) are as follows. In a, wild type versus knockout: P < 0.0001 for all comparisons. In b, wild type versus knockout: P = 0.0192 (0 h, starved), P = 0.0004 (3 h, starved), P < 0.0001 for the other comparisons. In c, wild type versus knockout: P = 0.0017 (0 h + H2O2), P < 0.0001 for the other comparisons. In d, compared with wild type: P = 0.264 (HET, 0 h), P < 0.0001 for the other comparisons. In e, compared with wild type: P = 0.8121 (+/Q65P, 0 h), P = 0.545 (Q65P/Q65P, 0 h), P = 0.0935 (+/Q65P, 3 h), P = 0.2012 (Q65P/Q65P, 3 h), P < 0.0001 for the other comparisons. f, Clinical effect of the rs34311866 (p.M393T) variant in the human TMEM175 on longitudinal course of Parkinson’s disease. Longitudinal effects for carriers of no (blue), one (green), or two (red) p.M393T variants are shown for mixed-effects linear regression models without multiple testing correction; 95% confidence intervals are indicated by coloured bands. In the UPenn cohort, p.M393T carriers had faster cognitive decline (two-tailed P = 0.005, as captured by lower DRS-2 scores) and faster motor decline (two-tailed P = 0.032, higher UPDRS-3 scores). In the PPMI cohort, p.M393T carriers trended (two-tailed P = 0.066) towards faster motor decline, although no statistical significance was reached (presumably because of shorter observation period, of 5 years after diagnosis). As TMEM175 variants are associated with neurodegenerative diseases and AKT is known to protect neurons against stress-induced damage25,35,36, we tested whether lysoKGF protects neurons. To do this, we used a neuronal culture model in which damage induced by insults such as the neurotoxin MPTP, H2O2 and nutrient removal can be quantified well37–39. MPTP interferes with mitochondrial metabolism, inducing parkinsonism in humans and in animal models84. Consistent with previous findings85,86, incubating midbrain neurons with MPP+ (the active form of the neurotoxin) led to perturbations and beading of neuronal processes in a dose-dependent manner. When the size of such damaged areas normalized to the total neuronal area (that is, damage index) was quantified, TMEM175-knockout neurons exhibited much more severe damage compared to wild type (a). Both nutrient removal and reactive oxygen species (such as H2O2) cause neuronal damage39. Under each combination of H2O2 treatment after B27 removal, TMEM175-knockout neurons exhibited more severe damage than wild type (b, c). Growth factors such as insulin are known to mitigate neuronal damage but the mechanisms are not well-understood87. In wild-type neurons, insulin reduced the damage induced by B27 removal and/or H2O2 treatment. Notably, this protection was nearly absent in TMEM175-knockout neurons (d), which suggests an essential role for TMEM175. In heterozygous neurons, the degree of damage was intermediate between that of wild-type and TMEM175-knockout neurons (d). Thus, Tmem175 gene dosage has an incremental effect on neuronal protection. Decreasing ITMEM175 by about 50% is sufficient to compromise the protection. In support of this finding, neurons from TMEM175(I390T) knock-in mice were also more prone to MPP+-induced damage than were neurons from wild-type mice (Extended Data Fig. 8c). We also tested whether p.Q65P provides additional protection. When challenged with B27 deprivation and H2O2, neurons from both heterozygous (Q65P/+) and homozygous (Q65P/Q65P) mice had much less damage than those from wild type (e). The difference was even more notable between TMEM175(Q65P)-carrying neurons and TMEM175-knockout neurons. For example, after a 14-h starvation the TMEM175-knockout neurons had 27% damage and wild-type neurons had 15%—but those from Tmem175Q65P/+ mice had only 6%. Similarly, H2O2-induced damages were mitigated in TMEM175(Q65P)-carrying neurons (c, e). These data suggest that p.Q65P is neuroprotective and constitutes a potential mechanism that underlies a reduced susceptibility to developing Parkinson’s disease.