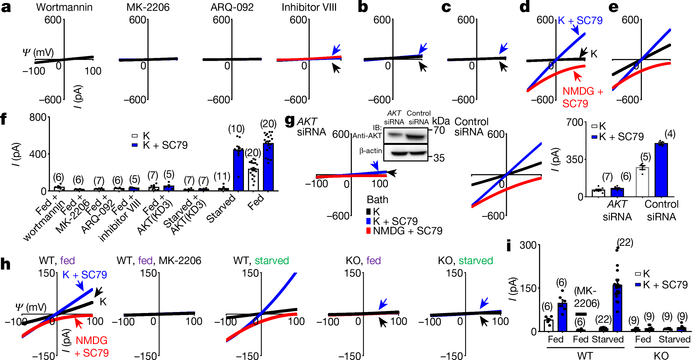
Fig. 2 |. AKT is necessary and sufficient to activate TMEM175.
a–f, Currents from TMEM175-transfected HEK293T cells. In b, c, a dominant-negative AKT (triple-mutant AKT(K179M/T308A/S473A); AKT(KD3)) was cotransfected. In a, cells were pretreated with wortmannin (20 μM, 2 h), an allosteric AKT inhibitor (MK-2206 (20 μM, 3 h) or ARQ-092 (20 μM, 3 h)) or AKT inhibitor VIII (10 μM, 3 h) before lysosomal dissection for recording. In a, b, e, cells were not starved; in c, d, cells were starved in Hank’s balanced salt solution (HBSS) medium for 2 h. g, Currents from TMEM175-transfected cells without starvation pretreated with small interfering (si)RNAs against AKT1, AKT2 and AKT3 or with a control siRNA. Inset, western blot showing the efficient knockdown of AKT. For gel source data, see Supplementary Fig. 1. IB, immunoblot. h, i, Currents from wild-type and TMEM175-knockout mouse hippocampal neurons before or after starvation (overnight in DMEM). The AKT activator SC79 (10 μM) was applied to the bath during some of the recordings (blue and red traces). Bar graphs in f, g, i show averaged current sizes (at 100 mV). Data are mean ± s.e.m. Numbers of recordings are in parentheses. Arrows are used to indicate curves that overlap and are not easily distinguished. Colours denote conditions for recording: from a bath containing K+ (black), K+ and SC79 (blue) or NMDG and SC79 (red).