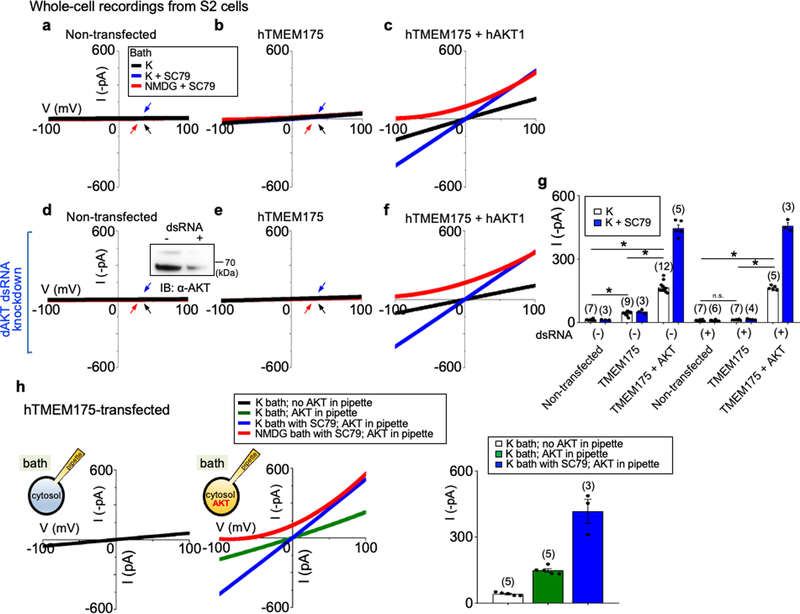
Extended Data Fig. 2 |. AKT is required for the reconstitution of human TMEM175 as a functional ion channel.
a–f, Whole-cell currents were recorded from Drosophila S2 cells without (a–c, h) or with (d–f) endogenous Akt (‘dAKT’) knocked down using dsRNA treatment. a, d, Nontransfected S2 cells. b, e, h, S2 cells transfected with human TMEM175 alone. c, f, S2 cells cotransfected with human TMEM175 and human AKT1. A western blot showing the reduction of Drosophila Akt protein by dsRNA treatment is shown in the inset in d. For gel source data, see Supplementary Fig. 1. g, Summary of current amplitudes (at −100 mV, recorded in K+-containing bath with or without SC79). Recordings were done using a ramp protocol (−100 mV to 100 mV in 1 s, Vh = 0 mV). Bath solution contained 150 mM K+, 150 mM K+ with SC79 (an activator of human AKT) or 150 mM NMDG with SC79, as indicated. In a, b, d, e, arrows are used to indicate curves that overlap and are not easily distinguished. Black, recorded from bath containing K+; blue, recorded from bath containing K+ and SC79; red, recorded from bath containing NMDG and SC79. Data are mean ± s.e.m. Numbers of recordings are in parentheses. In g, *P ≤ 0.05 (compared with those from cells cotransfected with human TMEM175 and human AKT). P values (unpaired two-tailed t-tests) are as follows: without dsRNA treatment, P < 0.0001 for TMEM175 vs nontransfected, TMEM175+AKT vs nontransfected and TMEM175+AKT vs TMEM175; with dsRNA treatment, P = 0.1253 for TMEM175 vs nontransfected, P < 0.0001 for TMEM175+AKT vs TMEM175 and TMEM175+AKT vs nontransfected. h, Whole-cell currents recorded from S2 cells transfected with human TMEM175 alone, with (middle) or without (left) recombinant human AKT1 protein (1 μg ml−1) included in the pipette solution. Right, summary of current amplitudes (at −100 mV; b and g show an additional control without AKT protein in pipette. We further tested the necessary and sufficient role for AKT in ITMEM175 by reconstituting TMEM175 in the Drosophila S2 cell line, which does not have endogenous TMEM175. In HEK293T cells, cotransfection of AKT1 did not further increase ITMEM175 (Extended Data Fig. 6a), consistent with the idea that the three mammalian AKTs are already abundantly expressed in the cells79. S2 cells do not have TMEM175 (which is absent in insects), and have only one Akt gene, making it easier to knock down. In addition, the Drosophila Akt (dAKT) protein is not well-conserved with the mammalian AKTs (61% identity to human AKT1) and is less likely to support human TMEM175 function. For technical reasons, we were unable to enlarge S2 cell lysosomes suitable for patch-clamp recording. We therefore recorded whole-cell currents as an assay for functional channels. Transfecting human TMEM175 alone into S2 cells generated little current (about −20 pA at −100 mV) above the level of endogenous K+ currents (a, b, g). In addition, knocking down Drosophila Akt with dsRNA55 completely eliminated the small ITMEM175 (d, e, g). Cotransfecting human TMEM175 with a human AKT1 (hAKT1), however, led to robust ITMEM175, either with or without the endogenous Drosophila Akt knocked down (c, f, g). The currents were potentiated by SC79 in human AKT cotransfected cells but not in those transfected with TMEM175 alone, supporting the notion that SC79 acts on mammalian AKTs to open the channel. We finally tested whether purified recombinant AKT protein is sufficient to activate TMEM175. In S2 cells transfected with human TMEM175 alone (which had minimal current), application of SC79 into the bath increased the currents to −417.0 ± 53.9 pA (h) (n = 3, −100 mV) when human AKT1 protein was introduced into cytosol via pipette solution dialysis. Together, our data suggest that AKT is obligatory for a functional mammalian TMEM175 channel.