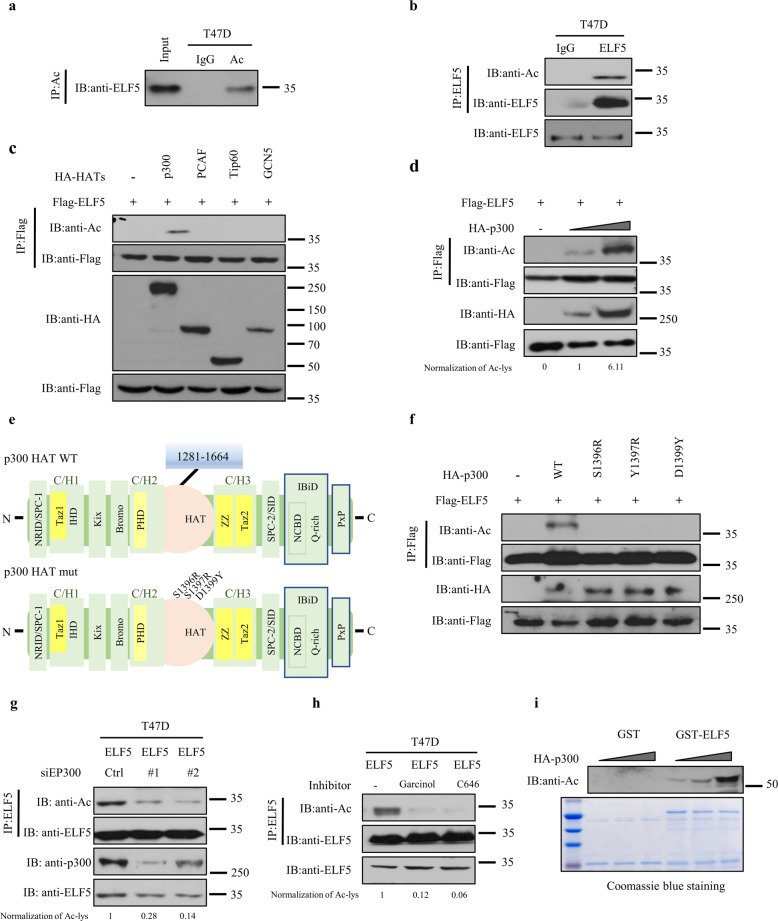
Fig. 2. Acetylation of ELF5 by acetylatyltransferase p300.
Acetylation of endogenous ELF5. a Lysates of T47D cells was immunoprecipitated with an anti-acetylated-lysine (AcK) antibody (Ab) or normal IgG, followed by western blot performed with anti-ELF5 Ab. b Lysates of T47D cells was immunoprecipitated with an anti-ELF5 Ab or normal IgG, followed by western blot performed with anti-AcK Ab. c p300-mediated acetylation of ELF5. HEK293T cells were co-transfected with Flag-ELF5 and different HA-tagged HATs, HA-p300, HA-PCAF, HA-GCN5, or HA-Tip60, and cell lysate was immunoprecipitated with anti-Flag affinity gel and immunoblotted with the anti-AcK Ab. d p300-mediated acetylation of ELF5 in a dose-dependent manner. HEK293T cells were co-transfected with Flag-ELF5 and increasing amounts of HA-p300. After 48 h of transfection, the cell lysates were subjected to co-immunoprecipitation with anti-Flag Ab and immunoblotted with the anti-AcK Ab. e Top: A model for full-length p300 showing all the different domains. It was compiled based on several recent analyses, and the catalytic acetyltransferase domain corresponding to residues 1281–1664. Bottom: A model for full-length p300 with single substitutions that inactivate acetyltransferase catalytic activity. f Acetylation of ELF5 by p300 depended on its intrinsic KAT activity. HEK293T cells were co-transfected with Flag-ELF5 and p300 or its catalytic mutant, and the cell lysate was subjected to co-immunoprecipitation with anti-Flag affinity gel and immunoblotted with the anti-AcK Ab. g Effect of p300 knockdown on the acetylation of ELF5. T47D cells were transfected with siRNA targeting EP300 and then immunoprecipitated with an anti-ELF5 antibody, followed by western blot with anti-AcK Ab. h Effect of ELF5 acetylation in the presence of p300 inhibitors. T47D cells were treated without or with Garcinol (10 μM), or C646 (10 μM) for 6 h and then immunoprecipitated with an anti-ELF5 Ab or normal IgG, followed by western blot with anti-AcK Ab. i Acetylation of ELF5 by p300 as detected by in vitro acetylation assay. GST-ELF5 fusion protein was affinity-purified and quantified by in vitro acetylation assay. The reaction mixture was subjected to SDS-PAGE and immunoblotted with anti-AcK Ab. Purified GST-ELF5 was examined by Coomassie blue staining.