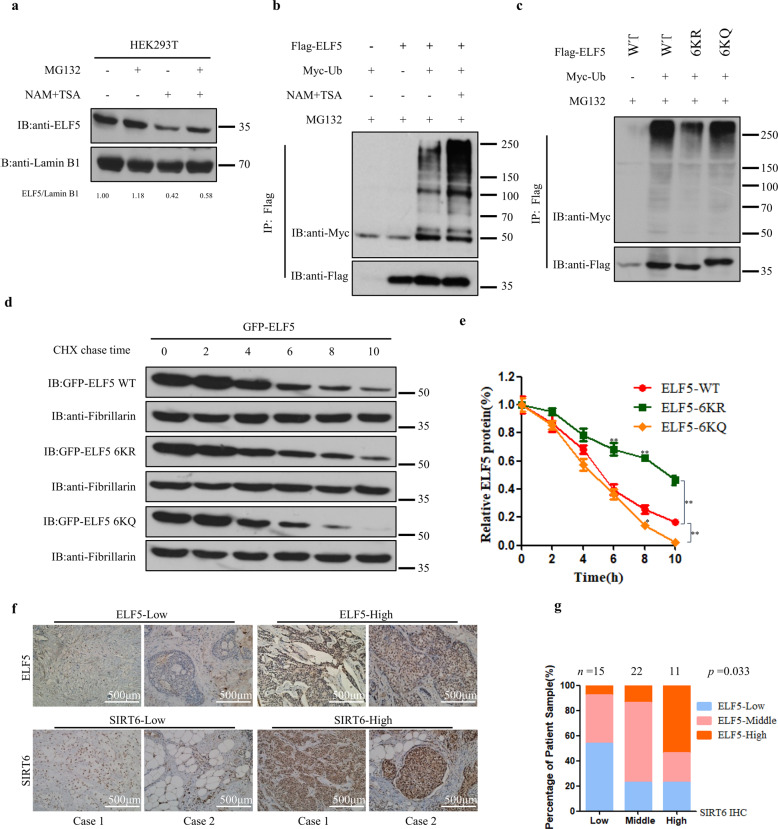
Fig. 5. Acetylation promotes the proteasome-mediated degradation of ELF5.
a Inhibition of deacetylation and protein synthesis on the protein level of ELF5. HEK293T cells were treated without and with 2 μM TSA and 5 mM NAM in the absence or presence of 20 μM MG132. Endogenous ELF5 was probed by anti-ELF5 Ab. b NAM- and TSA-mediated regulation of the ELF5 ubiquitination. Ubiquitination of Flag-ELF5 expressed in HEK293T cells the absence and presence of NAM + TSA with MG132. c Quantitation of the ubiquitination levels of Flag-ELF5 WT, Flag-ELF5 6KR, and Flag-ELF5 6KQ in HEK293T cells. d, e Half-lives of ELF5 and mutants. HEK293T cells expressing GFP-ELF5-WT, GFP-ELF5-6KR, or GFP-ELF5-6KQ were treated with 50 μM Cycloheximide, and the expression levels of these proteins were analyzed by western blot with anti-GFP Ab. The graph shown represents the ELF5 half-lives in Fig. 5e. Data are the means ± SDs from three determinations. *p < 0.05; **p < 0.01. f, g The expression of ELF5 and SIRT6 in human breast cancer tissues. Representative images tissue samples from breast cancer patients showing the expression levels of ELF5 and SIRT6 as assessed by immunohistochemistry. Both ELF5 and SIRT6 levels were classified as low, medium, or high based on the intensities of their IHC staining, and the percentages of patients classified in each category are depicted in the histogram in Fig. 5g.