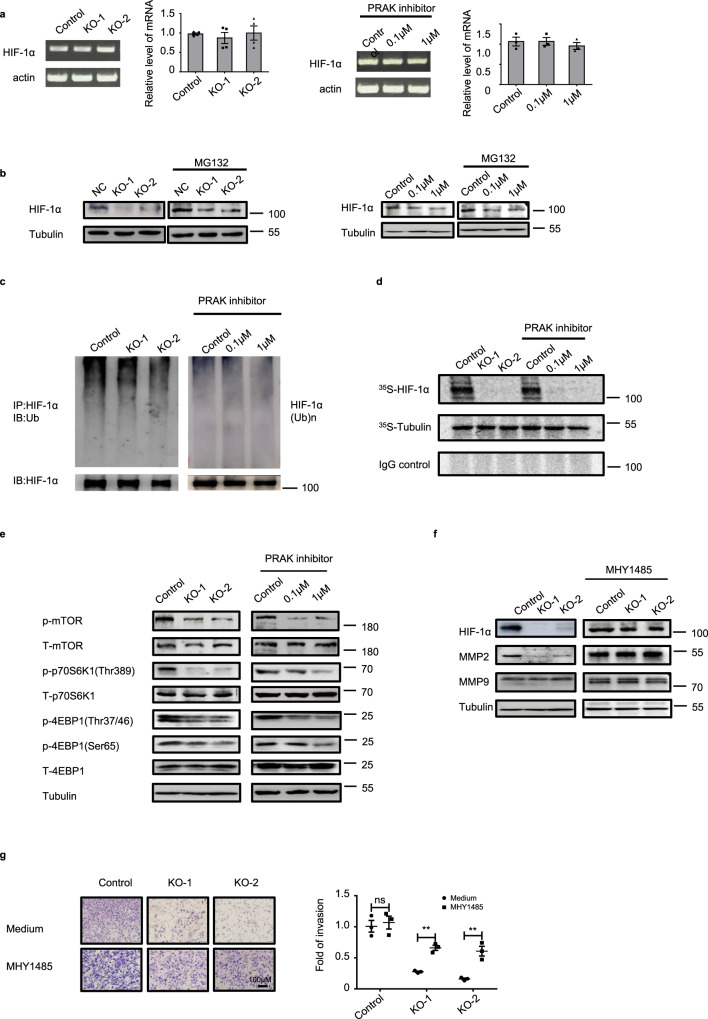
Fig. 5. PRAK promotes HIF-1α translation by regulating mTORC1 activity.
a HIF-1α mRNA levels in Prak+/+ and Prak−/− B16 cells (left) and in B16 cells treated with the PRAK inhibitor (right) as measured by real-time RT-PCR. An agarose gel of the PCR products is shown on the Left. b HIF-1α protein levels in parent, Prak−/− and GLPG0259-treated B16 cells in the absence or presence of MG132 as determined by Western blotting. Tubulin served as a loading control. c B16 cells were cultured in the presence of MG132 for 10 h. HIF-1α was precipitated from the lysate and the blot was probed with anti-ubiquitin antibodies. d B16 cells were cultured in the presence of CoCl2 with the addition of 500μCi of [35S]-L-methionine and [35S]-L-cysteine for 6 h. Immunoprecipitation was performed with antibodies against HIF-1α or tubulin or with control IgG. Newly synthesized proteins of the expected size were detected by autoradiography. e Cell lysate was prepared from control, Prak knockout, and inhibitor-treated B16 cells. The blot was probed with anti-phospho- or total mTOR, S6K1 and 4EBP1. Tubulin served as a loading control. f, g Prak+/+ and Prak−/− B16 cells were cultured with or without the mTORC1 agonist MHY1485 (1 μM for 12 h). HIF-1α, MMP2, and MMP9 expression was detected by immunoblotting (f). The invasive capacity of these cells was analyzed using Matrigel assay (g). Each experiment was repeated at least three times with similar results. ns no significant, **p < 0.01. p-value was determined by a two-tailed, unpaired t-test.