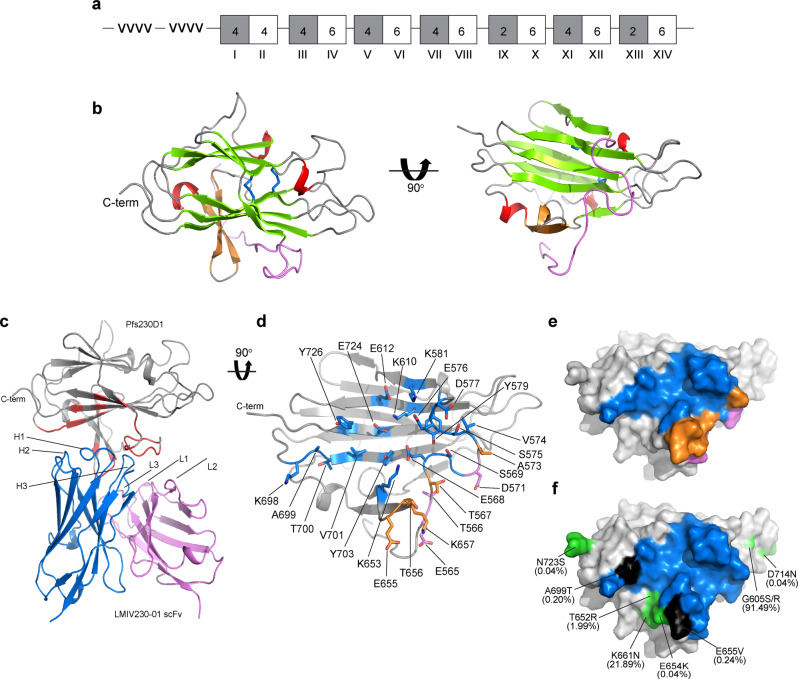
Fig. 3. Structural definition of LMIV230–01 scFv epitope in Pfs230D1.
a Domain organization of Pfs230D1. A-Type (gray) and B-Type (white) 6-Cys domains are shown in boxes. Numbers inside the boxes indicate the number of conserved cysteines. Domain numbers are labeled in Roman numerals. -vvvv- indicates a highly repetitive region that is cleaved from the mature protein. b Structure of Pfs230D1. N-terminal loop in violet, beta-sandwich in green, beta strands in orange, helices in red, loops in gray and disulfide bonds in blue sticks. c Overall structure and epitope for the Pfs230D1–LMIV230–01 scFv complex. Pfs230D1 in gray; LMIV230–01 scFv heavy chain in blue; LMIV230–01 scFv light chain in violet; epitope in red. d Orthogonal detailed view of the Pfs230D1 epitope for LMIV230–01 scFv. Pfs230D1 in gray ribbon; residues contacted by LMIV230–01 scFv heavy chain, light chain and both are highlighted in blue, violet, and orange, respectively. e Surface representation of the epitope on Pfs230D1. The orientation and the color scheme are the same as in (b). f Polymorphisms in Pfs230 mapped onto the surface of Pfs230D1. Orientation as in (c). The LMIV230–01 scFv binding epitope in blue, polymorphic residues are in green, polymorphic residues within the epitope are in black. The frequency of the polymorphisms is in parentheses.