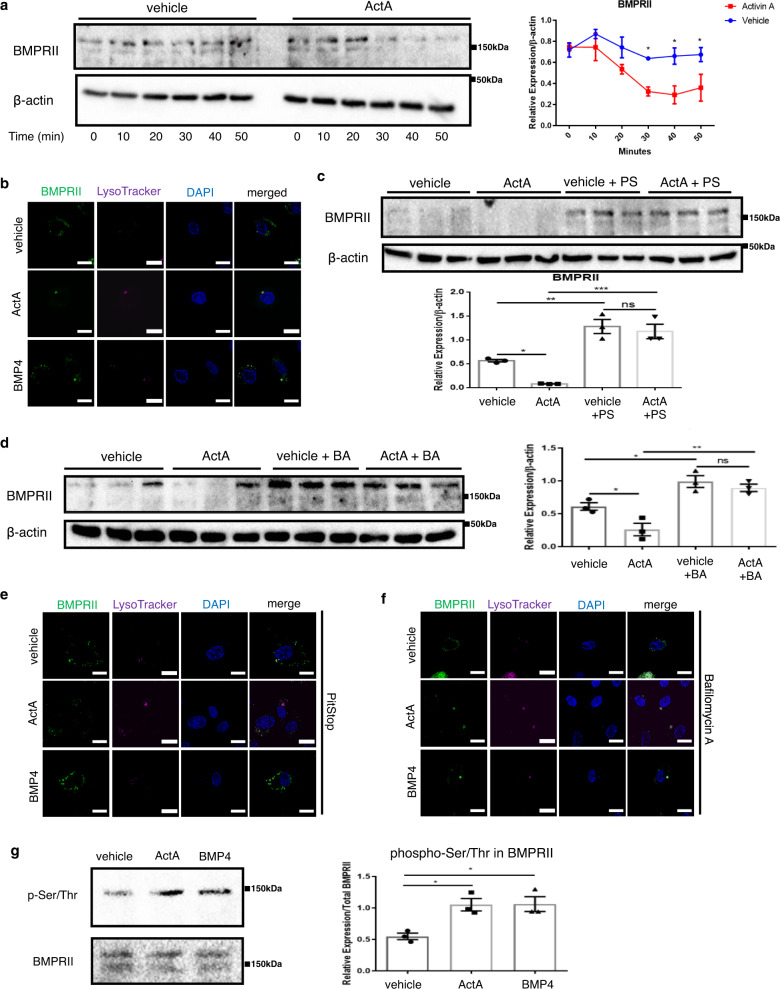
Fig. 4. ActA accelerates ligand-mediated BMPRII endocytosis and lysosomal degradation.
a Cycloheximide-chase assay for BMPRII in PAECs treated with either vehicle or ActA (20 ng/mL) (n = 3 biologically independent cells in each group). b Representative immunocytochemistry images for GFP-tagged BMPRII (in green color) in PAECs treated with either vehicle, BMP-4 (20 ng/mL), or ActA (20 ng/mL) for 30 min. Lysosomes were visualized using LysoTracker (in magenta color). Bars: 20 μm. Similar results were obtained in three independent experiments. c, d Immunoblots and densitometric analysis for BMPRII in PAECs pretreated with either PitStop (PS) (c) or bafilomycin A (BA) (d) for 2 h, followed by treatment with either vehicle or ActA for 30 min in the presence of cycloheximide (n = 3 biologically independent cells in each group). e, f Immunocytochemistry for GFP-tagged BMPRII (in green color) and lysosomes (in magenta color) in PAECs pretreated with either PitStop (e) or bafilomycin A (f), followed by treatment with either vehicle, BMP-4, or ActA. Bars: 20 μm. Similar results were obtained in four independent experiments. g Representative immunoblots and densitometric analysis for serine/threonine-phosphorylated BMPRII in PAECs treated with vehicle, BMP-4, or ActA (n = 3 biologically independent cells in each group). ***P < 0.001; **P < 0.01; *P < 0.05. Exact P values are shown in the Source data file. Data are presented as the mean ± SEM. Two-sided Student’s t-test was used to analyze the differences between the vehicle and ActA-treated groups at each time point (a). One-way ANOVA with Tukey’s post hoc test for multiple comparisons was used to analyze the differences between each study group in all of the western blot quantitation procedures (c, d, and g).