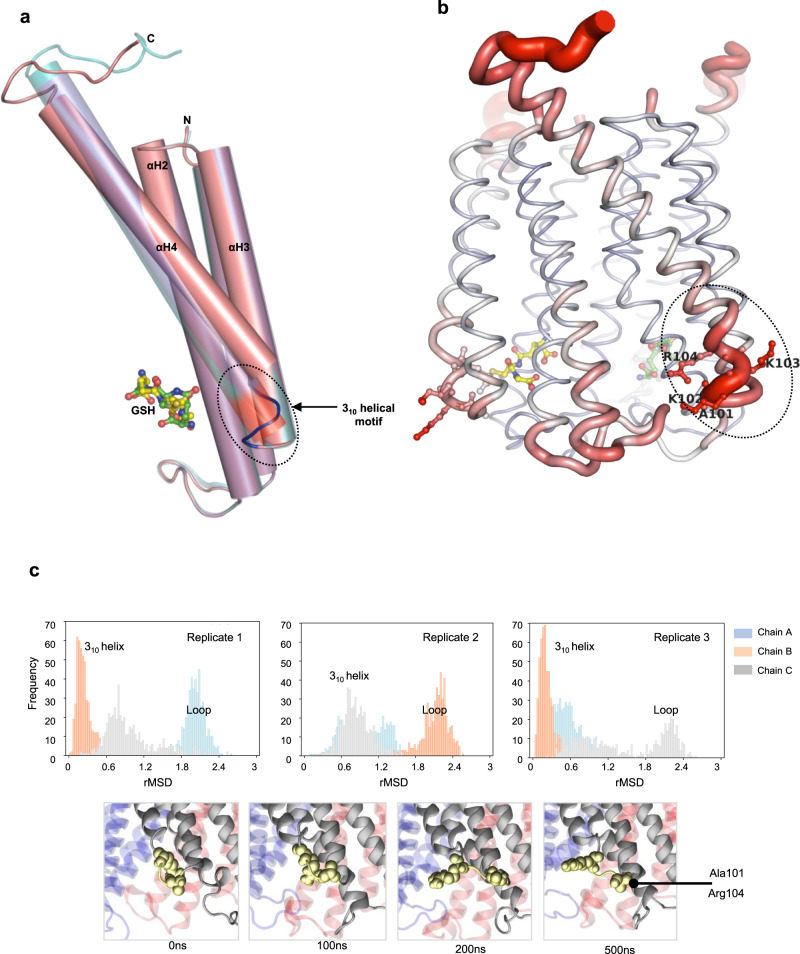
Fig. 5. Structural asymmetry of MGST2.
a Superimposition of chain A (translucent teal) and chain C (translucent slate) on chain B (opaque salmon) of holo-MGST2 in cylindrical rendering. Note the loop conformation (blue) of the 310 helical motif in chain B where GSH is not bound, whereas this motif exists in a 310 helix conformation (red) in chain A and C where GSH is bound. b B-factor putty representation of holo-MGST2 structure focusing on loop conformation of the 310 helical motif at the active site without GSH. Dark red color with wider tube indicates regions with higher B-factor. c Stability of 310 helical motif in apo-MGST2 MD simulations. RMSD distribution for three 500 ns MD replicate simulations. Unfolding of the 310 helix happens only at one of the subunits at a time for the apo state as shown by rMSD increase in one chain every time (top panels, compare with Supplementary Fig. 8) and involves breaking of the H-bond between Ala101 and Arg104 (bottom panels) along with specific inter-subunit contacts with the neighboring subunit (see further details in Supplementary Fig. 8).