Figure 4.
INTS4 recruitment to the cleavage module and its scaffolding role in organizing mobile domains of the complex
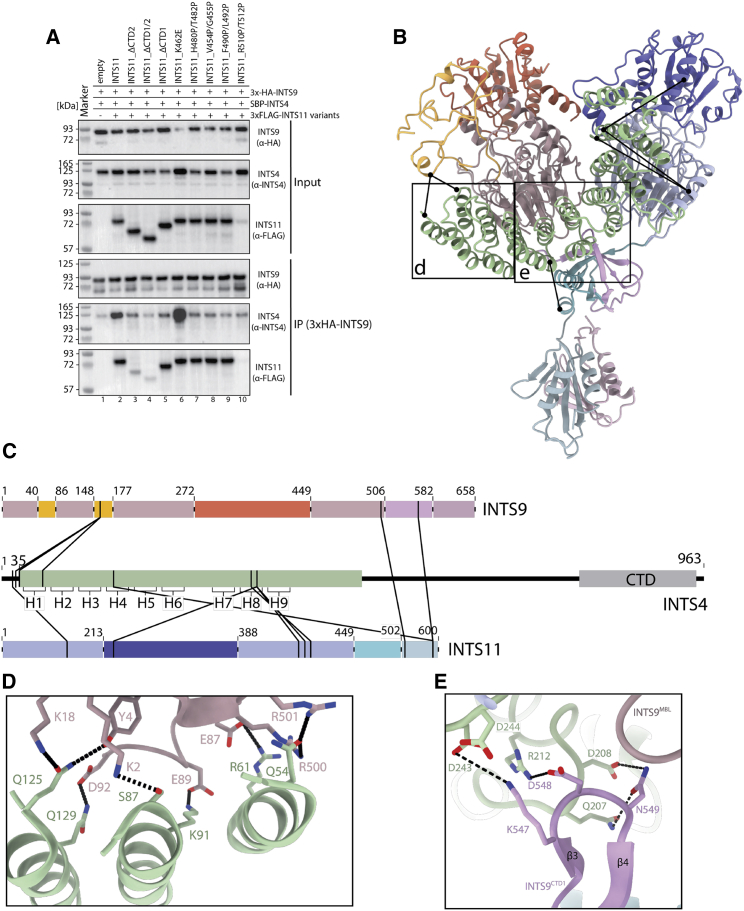
(A) Western blot showing the results of the HA-agarose pull-down experiments from HEK293T cells co-expressing the 3×HA-INTS9, SBP-INTS4, and 3×FLAG-INTS11 variants. Deletion of CTD2 or CTD1/2 (lanes 3 and 4) as well as point mutations in CTD1 (lanes 7–9) result in reduced recruitment of INTS4 compared with wild-type (WT) INTS11 (lane 2). INTS11K462E (lane 6) was not meant to disrupt CTD1 and recruitment of the INTS4. Elevated levels of INTS4 in this pull-down likely originate in higher INTS4 levels already in the input sample and/or additional electrostatic interactions between newly introduced 462E and the neighboring positively charged surface of INTS4.
(B) Model of the cleavage module, showing intermolecular cross-links with a distance of less than 35 Å.
(C) Cross-linking and mass spectrometry (XL-MS) data mapped along the sequence of each protein.
(D and E) Interactions of the INTS4NTD with the nuclease heterodimer.