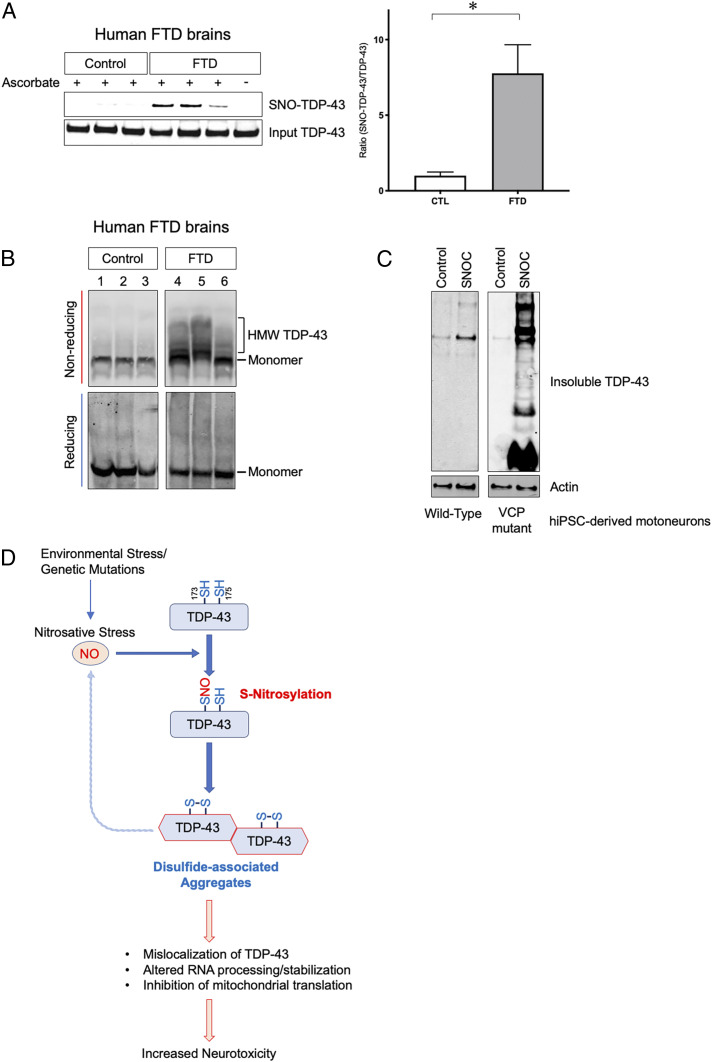
Fig. 6.
S-nitrosylation and aggregation of TDP-43 in human FTD brains and hiPSC-derived motoneurons. (A) Detection of SNO-TDP-43 by biotin-switch assay in human postmortem brain lysates prepared from control (n = 9) and FTD patients (n = 13) (SI Appendix, Fig. S5 A–C and Table S1). Graph indicates mean + SEM; *P = 0.0080 by two-tailed Student’s t test. (B) HMW TDP-43 disulfide species in human FTD brains. Brain lysate was separated by SDS-PAGE under reducing or nonreducing conditions, and immunoblotted with anti-TDP-43 antibody. (C) SNOC increases accumulation of insoluble TDP-43 in motor neurons derived from hiPSCs. hiPSCs carrying a mutation in the VCP gene were differentiated into motor neurons and exposed to 100 µM SNOC. Insoluble fractions were analyzed by immunoblot. (D) Proposed schema for S-nitrosylation-induced TDP-43 aggregation and neurotoxicity. In neurodegenerative diseases such as ALS/FTD, increased nitrosative stress engenders S-nitrosylation of TDP-43 at Cys173 or Cys175, facilitating intramolecular disulfide bond formation. This reaction contributes to oligomerization/aggregation of TDP-43. Aggregated TDP-43 is cleared from the nucleus and mislocalizes to cytoplasmic SGs and mitochondria. This causes a further increase in NO generation and alterations in RNA processing, stabilization, or translation, contributing to TDP-43 neurotoxicity.