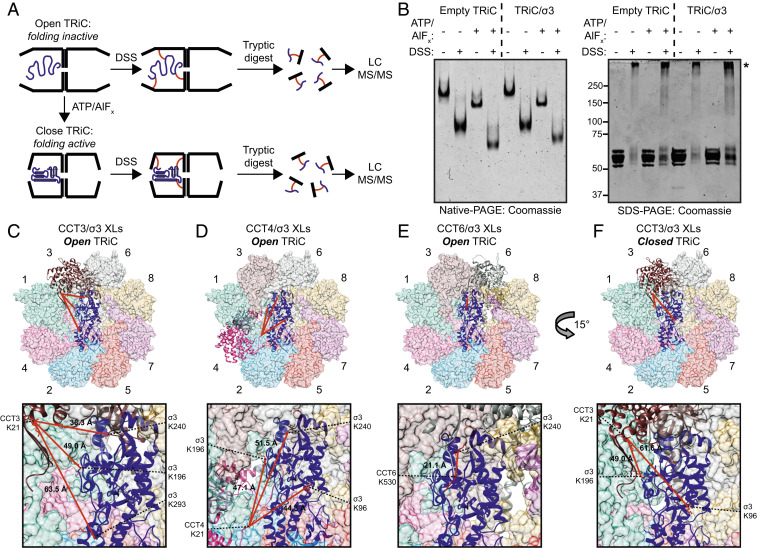
Fig. 4.
Cross-linking mass spectrometry reveals changes in the conformational mobility of the σ3 substrate in different states of TRiC cycling. (A) Workflow of the XL-MS approach. (B) Coomassie-stained native- (Left) or SDS- (Right) PAGE of TRiC/σ3 incubated with the conditions shown. Asterisk denotes cross-linked complexes. (C–F) TRiC/σ3 intermolecular cross-links identified in the open (C–E) and closed (F) conformations mapped onto the TRiC/σ3 structural model. Expanded views are shown below. Lysine residues participating in cross-links are colored red, and Cα–Cα cross-linking distance is listed.