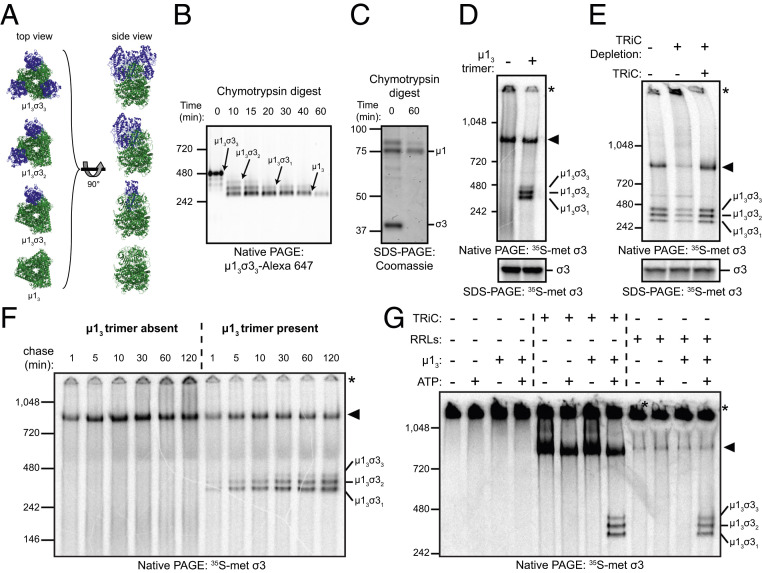
Fig. 5.
The TRiC chaperonin catalyzes the folding and assembly of σ3 onto the μ13 trimer base. (A) Ribbon tracings of μ1/σ3 assemblies (Protein Data Bank: 1JMU). σ3 monomers are colored blue, and the µ13 trimer is colored green. (B) Native-PAGE of µ13σ33 digested for the intervals shown with chymotrypsin. (C) Coomassie-stained SDS-PAGE of purified µ13σ33 digested for the intervals shown. (D) Native- (Top) and SDS- (Bottom) PAGE of σ3 in vitro translation reactions incubated in the presence or absence of the µ13 trimer. (E) Native- (Top) or SDS- (Bottom) PAGE of σ3 in vitro translation reactions conducted in mock-depleted (−) or TRiC-depleted (+) RRLs with or without addition of purified TRiC (final concentration: 0.2 μM). (F) Native-PAGE of σ3 translated in RRLs for 2 min with [35S]methionine and incubated with cold methionine for the intervals shown in the presence or absence of µ13. (G) Native-PAGE of denatured [35S]methionine-labeled σ3 incubated with the conditions shown.