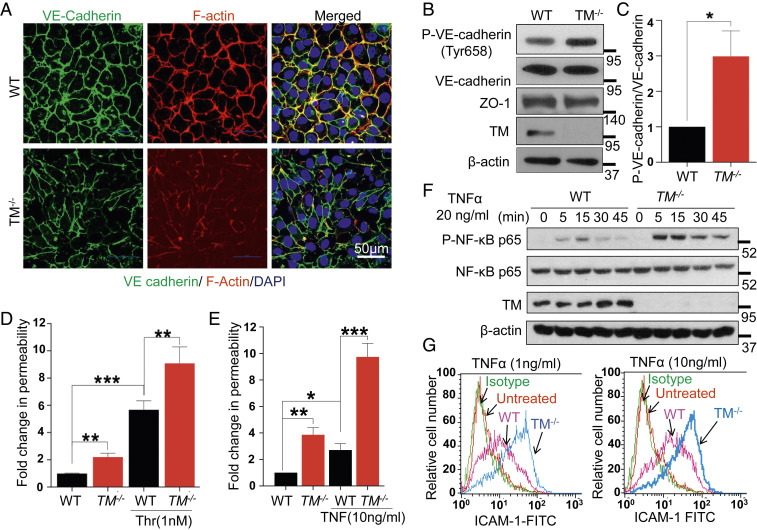
Fig. 1.
Deficiency of TM leads to endothelial cell dysfunction. (A) Confluent WT and TM−/− cells were fixed, permeabilized, and stained with rabbit anti‐VE-cadherin antibody and Alexa Fluor 488–conjugated goat anti‐rabbit IgG. F-actin was stained with Alexa Fluor 568–conjugated phalloidin followed by nucleus staining with Hoechst 33342. Immunofluorescence images were obtained with confocal microscopy. (Scale bar, 50 μm.) (B) Confluent WT and TM−/− cells were lysed, and levels of VE-cadherin (phosphorylated at Tyr658 and the total level), ZO-1, TM, and β-actin were analyzed by Western blotting. (C) Quantification (fold changes) in P-VE-cadherin (Y658) normalized to total VE-cadherin. Confluent WT and TM−/− cells were serum starved with basal medium containing 0.5% bovine serum albumin (BSA) for 3 h and then treated with either thrombin (1 nM) for 20 min (D) or TNF-α (10 ng/mL) for 4 h (E). The amount of Evans blue dye that leaked into the lower chamber in the Transwell assay plates was measured as described in Materials and Methods. (F) WT and TM−/− cells were treated with TNF-α (20 ng/mL) for various time points and levels of p65 NF-κB (phosphorylated and total); TM and β-actin were analyzed by Western blotting. (G) Cell surface levels of ICAM-1 in WT and TM−/− cells, treated with 1 and 10 ng/mL TNF-α for 4 h, were measured by flow cytometry. The untreated data belong to TM−/− cells. Data are mean ± SEM (n = 3). One-way ANOVA: *P < 0.05, **P < 0.01, and ***P < 0.001.