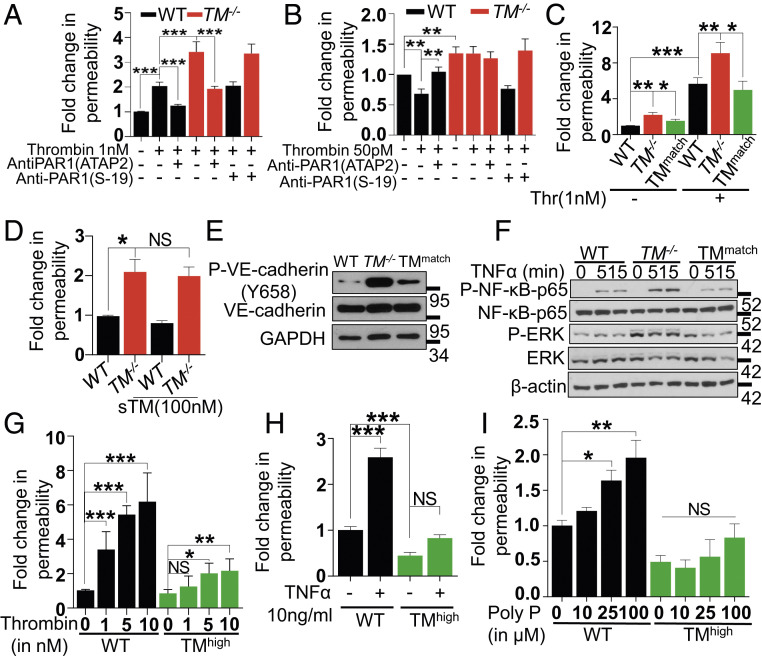
Fig. 2.
Thrombin-mediated PAR1 signaling in TM−/− cells with or without lentivirus-mediated TM transduction. (A) Confluent WT and TM−/− cells were serum starved for 3 h before the addition of PAR1 function-blocking (ATAP2) or nonblocking (S19) antibodies for 30 min. Permeability was induced with thrombin (1 nM) for 20 min. The amount of Evans blue dye that leaked into the lower chamber in the Transwell assay plates was measured as described in Materials and Methods. (B) Confluent WT and TM−/− cells were pretreated with the same antibodies as in A, followed by treatment with thrombin (50 pM) in the basal medium containing 0.5% BSA for 3 h. The amount of Evans blue dye that leaked into the lower chamber in the Transwell assay plates was measured as described in Materials and Methods. (C) Similar to A, except in addition to WT and TM−/− cells, TMmatch cells were used. (D) Confluent WT, TM−/−, and TMmatch cells were pretreated with sTM (100 nM) for 3 h before addition of Evans blue dye. (E) Confluent WT, TM−/−, and TMmatch cells were lysed, and levels of VE-cadherin (phosphorylated at Tyr658 and the total), TM, and GAPDH were analyzed by Western blotting. (F) Confluent WT, TM−/−, and TMmatch cells were treated with TNF-α (20 ng/mL) for 0, 5, and 15 min, and levels of p65 NF-κB (phosphorylated and total), ERK (phosphorylated and total), and β-actin were analyzed by Western blotting. (G) Confluent WT, TM−/−, and TMhigh cells were serum starved with basal medium containing 0.5% BSA for 3 h and then treated with various concentrations of thrombin (1 to 10 nM) for 20 min, TNF-α (10 ng/mL) for 4 h (H), and polyphosphate (10-100 µM) for 4 h (I). The amount of Evans blue dye that leaked into the lower chamber in the Transwell assay plates was measured as described in Materials and Methods. Data are mean ± SEM (n = 3). One-way ANOVA: NS, non significant, *P < 0.05, **P < 0.01, and ***P < 0.001.