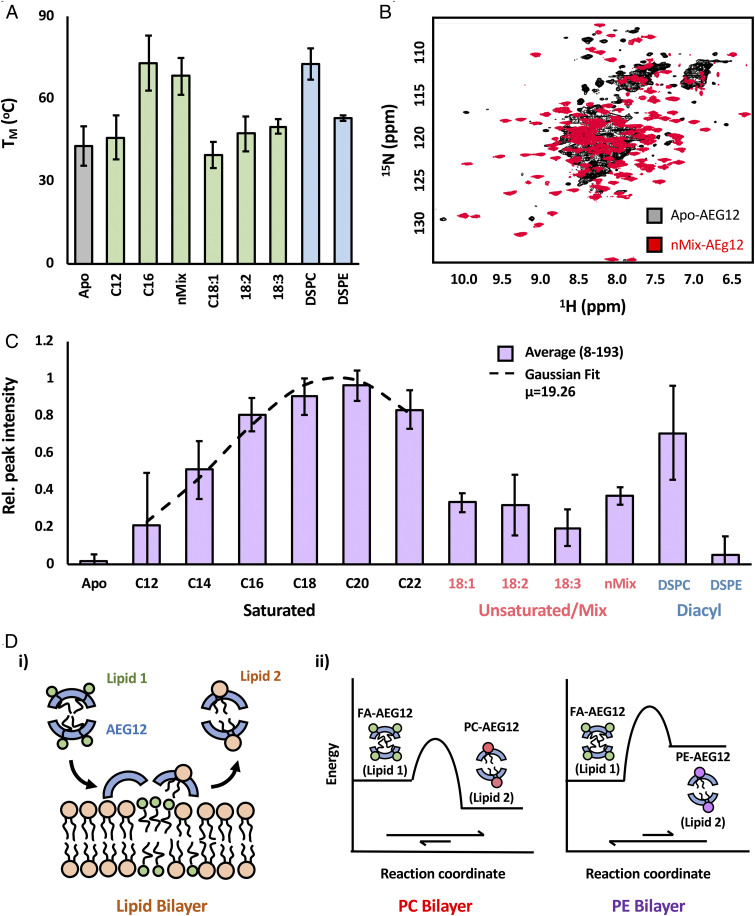
Fig. 6.
Thermodynamics of AEG12 ligand binding provides potential basis for cytotoxic selectivity. (A) Effect of ligand binding on AEG12 thermostability as assessed using circular dichroism. Representative CD spectra and melting curves shown in SI Appendix, Fig. S2. (B) 1H-15N-HSQC spectra of perdeuterated 15N-labeled AEG12. Apo-AEG12 (black) shows significant peak broadening indicative of intermediate (μs-ms) exchange dynamics. Loading AEG12 with long chain (nMix) fatty acids (red) significantly reduces peak broadening. 15N-HSQC peak intensity of 15N-labeled AEG12 loaded with a range of saturated and unsaturated fatty acids or diacyl chain ligands is shown in C. Relative peak intensities for AEG12 loaded with various hydrophobic ligands. Purple bars represent the average values across all peaks which could be confidently assigned and quantified, while the associated error bars represent the SD about this mean value. Data obtained from the saturated fatty acids could be fit to a Gaussian curve (dashed line) relative to acyl chain length, with a maximum peak intensity occurring at 19. Complete peak intensity values for all residues can be found in SI Appendix, Fig. S2. (D) Proposed model for AEG12-mediated cargo delivery. (i) General model illustrating the transfer of initial cargo (green) from AEG12 (blue) to the target bilayer. This is accompanied by the abstraction of phospholipids (orange) from said bilayer to occupy the otherwise empty AEG12 hydrophobic cavity. Cargo transfer is dependent on the relative stability of AEG12 in complex with Lipid 1 and Lipid 2, as illustrated by the free energy diagrams depicting the transfer of FA cargoes to PC and PE bilayers based on this model (ii).