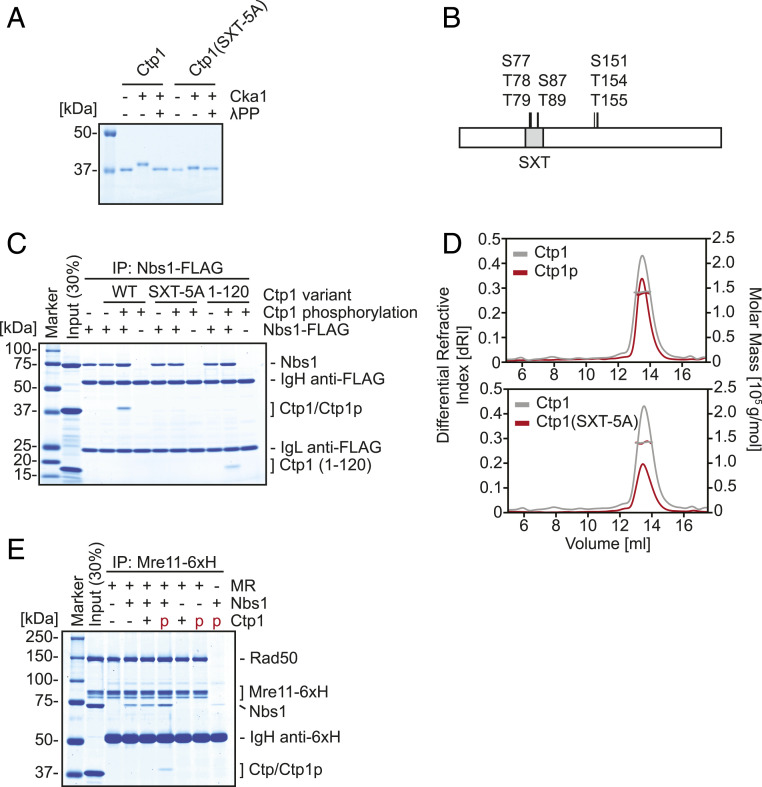
Fig. 1.
Phosphorylation of the SXT site of Ctp1 by Cka1 recruits Ctp1 to the MRN complex. (A) In vitro phosphorylation of Ctp1 variants by the CK2 catalytic subunit Cka1. (B) Summary of phosphorylation sites of Ctp1 revealed by mass spectrometry. (C) Interaction of Ctp1 variants and Nbs1 examined by coimmunoprecipitation with Nbs1-FLAG. (D) Molecular masses of phosphorylated Ctp1 and SXT-5A mutant were evaluated by SEC-MALS. Estimated molecular mass: Ctp1 (unmodified) = 142.0 kDa; phosphorylated Ctp1 = 138.5 kDa; and SXT-5A = 141.6 kDa. Theoretical molecular mass of unmodified Ctp1 monomer is 33.1 kDa. (E) MRN-Ctp1 complex formation and its requirement, examined by coimmunoprecipitation with hexahistidine-tagged Mre11 (Mre11-6xH).