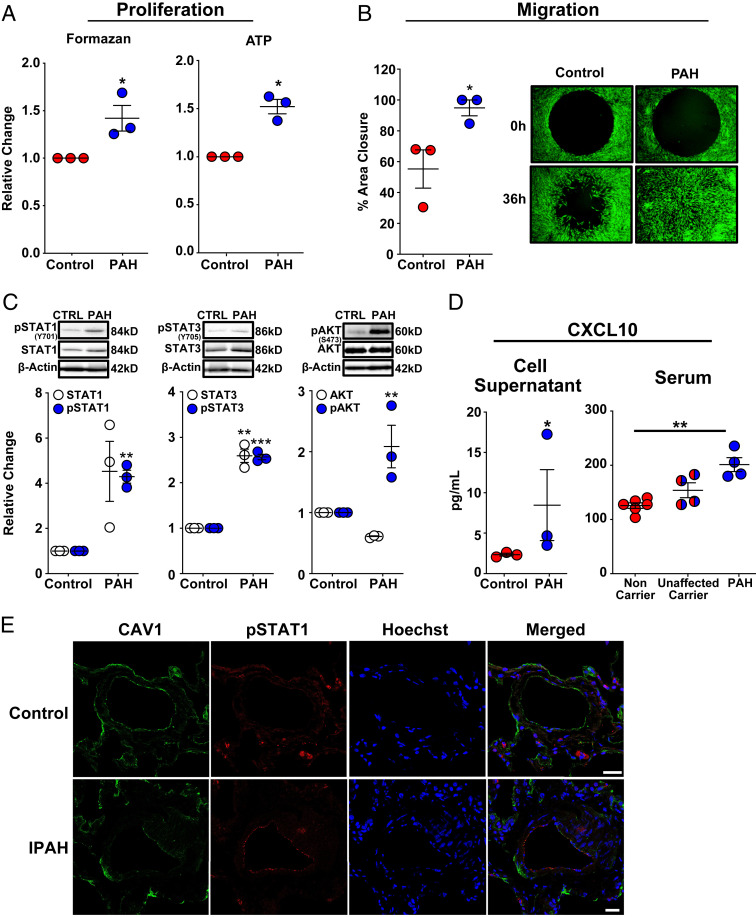
Fig. 5.
Dermal fibroblasts from patients with HPAH and the endothelial layer of distal pulmonary arterioles in patients with IPAH mirror CAV1-silenced primary human PAECs. (A) Dermal fibroblasts with a HPAH-associated CAV1 mutation demonstrated enhanced proliferation as assessed by MTS and ATP assays. Fibroblasts were seeded in 96-well plates in complete media for 6 h, then serum-starved for 24 h, followed by return to complete media for 72 h to assess proliferation. Data presented as mean FC ± SE. (B) Cell migration was significantly increased in CAV1-mutant fibroblasts from HPAH patients compared to healthy control fibroblasts. Fibroblasts were seeded in 96-well plates containing a circular gel insert (Oris Pro Cell Migration Assay) and allowed to adhere for 6 h. Cells were then serum-starved for 24 h, returned to complete media and the insert was removed. Percent closure of the monolayer defect was assessed 36 h later. Data presented as mean ± SE. A representative image at 0 and 36 h is shown (Right; 10× magnification). (C) CAV1-mutant fibroblasts from HPAH patients demonstrated a significant increase in phosphorylated (activated) STAT1, STAT3, and AKTSer473. Similarly, there was a trend toward an increase in total STAT1 expression (P = 0.06) and a significant increase in total STAT3. Phosphorylated and total STAT1 and STAT3 expression, as well as phosphorylated and total AKT, are normalized to β-actin. Densitometric quantification presented relative to control fibroblasts as mean ± SE, along with representative Western blots. (D) CAV1-mutant fibroblasts produced significantly higher concentrations of CXCL10 compared to control fibroblasts. CXCL10, measured by ELISA in cell supernatants after 48 h presented as mean ± SE. Replication used dermal fibroblasts from unique HPAH patients and healthy controls. HPAH patients harboring the identical CAV1 mutation (c.474delA) as the fibroblasts above had significantly higher serum CXCL10 concentrations than noncarriers. Notably, unaffected carriers of the CAV1 gene variant had a trend toward higher serum CXCL10 concentrations compared to noncarriers (P = 0.06), but similar levels compared to mutation carriers with HPAH (P = 0.29). CXCL10 was measured by ELISA and quantified based on a standard curve. Serum was available from two of the three HPAH patients that also provided dermal fibroblasts and two additional HPAH patients from PAH Biobank. *P < 0.05, **P < 0.01, ***P < 0.001. (E) Immunofluorescence staining revealed CAV1 (green) loss and STAT1 (red) activation in distal PAECs of IPAH patients (n = 5) compared to control lung samples (n = 5). Cell nuclei (blue) are stained with Hoechst 33342. (Scale bars, 25 µm.) Representative images are shown.