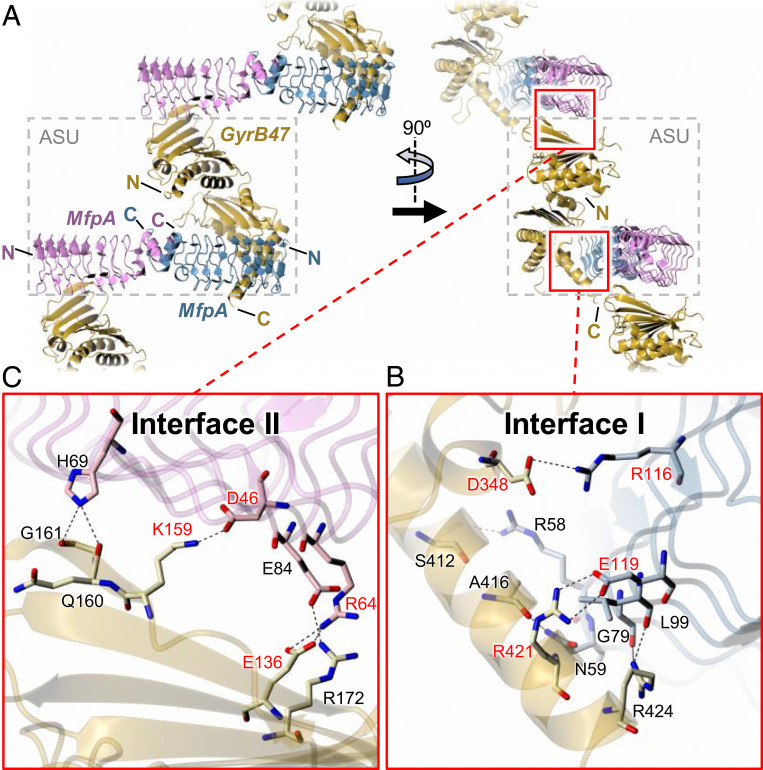
Fig. 5.
Structure of MfpA-MsGyrB47 complex. The Top panels (A) show orthogonal views of the crystal packing, which reveals a 2:1 MsMfpA:MsGyrB47 stoichiometry with two potentially significant interfaces between MsMfpA and MsGyrB47 that together create a zig-zag arrangement of the molecules. Interface I lies within the asymmetric unit (ASU; delineated by dashed gray box) and involves the C-terminal region of MsGyrB47 interacting with one half of the MsMfpA dimer. Interface II lies at the junction of two neighboring ASUs and involves the N-terminal region of MsGyrB47 interacting with the other half of the MsMfpA dimer. These interfaces are shown in detail in the two Insets, (B and C), below. Within each, there are both hydrogen bonds and salt bridges. We sought to probe the importance of these interfaces by disruption of two salt bridges in each interface through site-directed mutagenesis. The residues that were mutated are labeled in red. For clarity, in the Lower panels, the backbone traces are depicted as semitransparent ribbons.