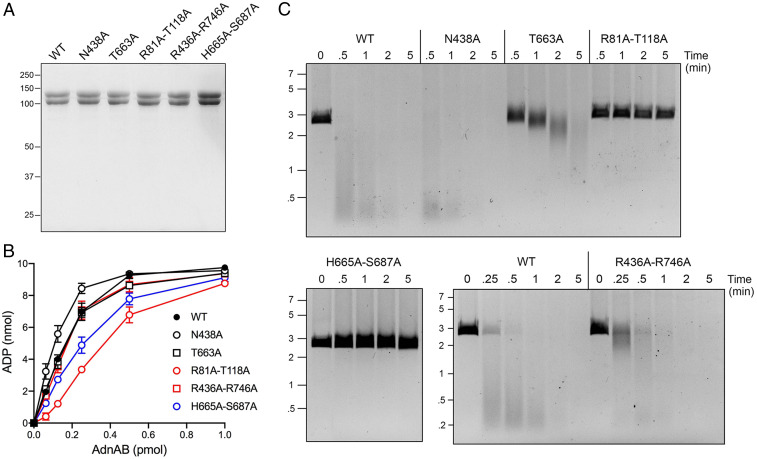
Fig. 3.
Effects of round 2 AdnAB mutations on DNA-dependent ATPase and DSB resection. (A) Aliquots (5 µg) of recombinant WT AdnAB and the indicated AdnB mutant heterodimers were analyzed by SDS-PAGE. The Coomassie blue-stained gel is shown. The positions and sizes (kDa) of marker polypeptides are indicated on the left. (B) ATPase reaction mixtures (10 µL) containing 20 mM Tris⋅HCl (pH 8.0), 2 mM MgCl2, 1 mM DTT, 1 mM (10 nmol) [α32P]ATP, 50 μM 85-mer ssDNA oligonucleotide, and AdnAB as specified were incubated for 10 min at 37 °C. The extent of ATP hydrolysis is plotted as a function of input AdnAB. Each datum in the graph is the average of three independent titration experiments ± SEM. (C) DSB resection reaction mixtures (50 μL) containing 20 mM Tris⋅HCl (pH 8.0), 2 mM MgCl2, 1 mM ATP, 200 ng linear pUC19 DNA (cut with SmaI), and 2.5 pmol of WT or mutant AdnAB were incubated at 37 °C. Aliquots (10 µL) were withdrawn and quenched at the times specified. The products were analyzed by agarose gel electrophoresis and staining with ethidium bromide. The positions and sizes (kbp) of linear duplex DNA markers are indicated on the left.