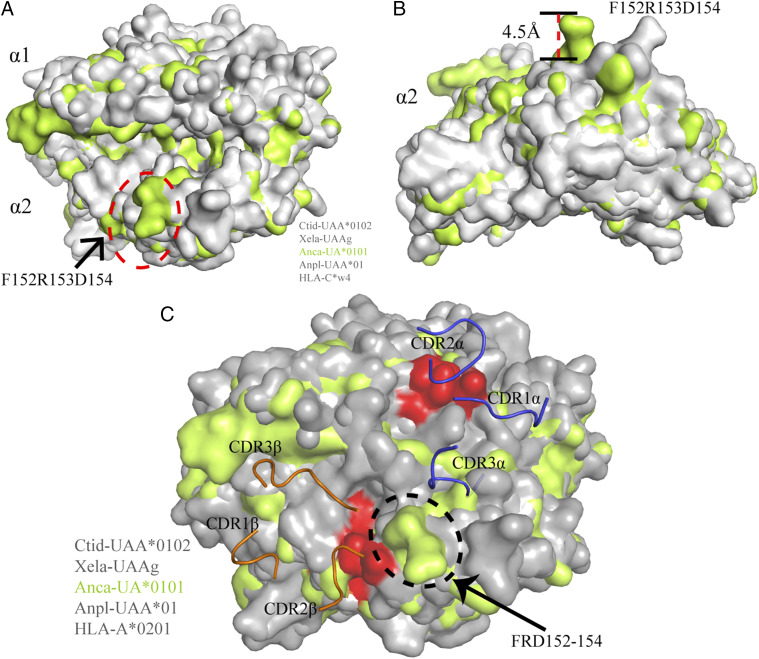
FIGURE 8.
The presence of 152F changes the upper point of pAnca-UA*0101. (A) The superimposition of the α1 and α2 domains of pAnca-UA*0101 (lime green) with other representative pMHC-Is (gray) is shown in surface representation. The floating region (152F-D154) in the pAnca-UA*0101 α2 domain is circled by an ellipse marked by red dotted line. (B) View of the α2 domain on the side; the side chain of the shifted region in the α2 domain of Anca-UA*0101 is ∼4.5 Å upwards of that in other representative pMHC-I molecules. (C) The structures of pCtid-UAA*0102, pXela-UAAg, pAnca-UA*0101, and pAnpl-UAA*01 were superimposed based on the structure of the HLA-A2-TCR complex (PDB ID: 1BD2). The key residues important for the interaction between pMHC-I and TCR in the PBG are colored in red. Residues 152F-R153-D154 potentially contact the TCR and are circled by an ellipse marked with black dots.