Figure 6.
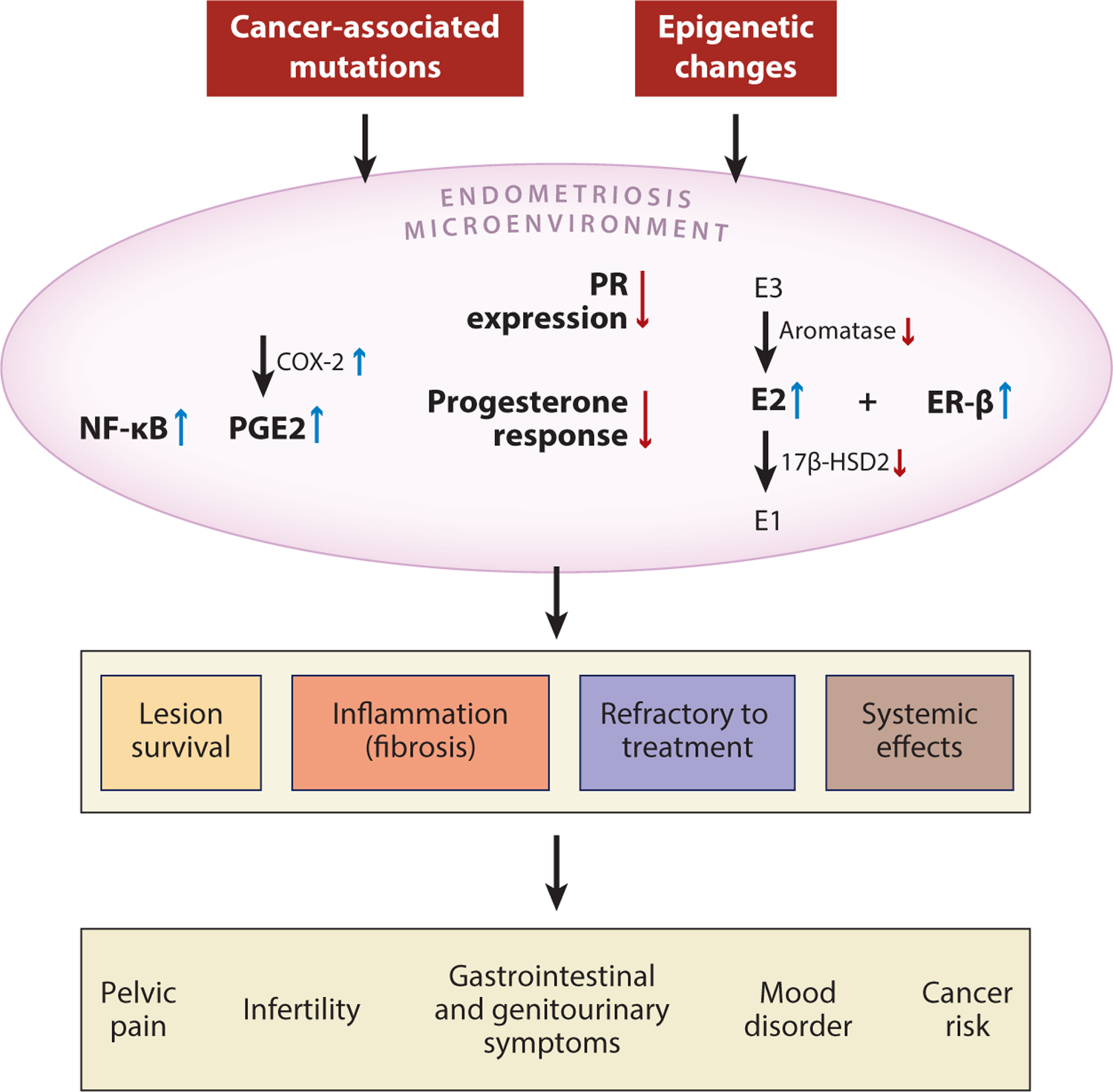
Summary of molecular changes involving inflammation and aberrant hormonal signaling in the pathogenesis of endometriosis. Both genetic and epigenetic alterations are associated with the development of endometriosis; these may include somatic mutations in cancer-associated genes or promoter methylation that alters the expression of genes involved in ER and PR signaling. As a result, the endometriosis microenvironment demonstrates numerous key molecular changes, including increased activity of NF-κB, PGE2, E2, and ER-β, that lead to inflammation and local dysregulation of hormonal pathways. As the chronic inflammation persists, it supports survival of the endometriotic lesion and induces local fibrosis. Persistent inflammation causes several systemic clinical effects and contributes to the refractory response to hormone-based treatment. Abbreviations: 17β-HSD2, 17β-hydroxysteroid dehydrogenase type 2; E1, estrone; E2, estradiol; E3, estriol; ER, estrogen receptor; NF-κB, nuclear factor-κB; PGE2, prostaglandin E2; PR, progesterone receptor.
