Abstract
Attention-deficit/hyperactivity disorder (ADHD) is a neurodevelopmental disorder associated with considerable impairment in psychiatric and functional domains. Although stimulant medication can reduce core symptoms of inattention, hyperactivity, and impulsivity, a subgroup of patients does not respond to this intervention. A precision medicine approach has been proposed, whereby biomarkers are used to identify an effective treatment approach for a given individual. This review synthesizes the existing literature on event-related potential (ERP) correlates of stimulant response in children diagnosed with ADHD, with the goal of evaluating the potential for ERP to inform precision medicine care in this population. Forty-three articles were examined and results tentatively suggest that stimulant medications normalize the amplitude of the P300 component, and this is also associated with behavioral improvement. In contrast, results generally indicate that stimulants do not significantly alter early processing components, although there are some exceptions to this finding. Implications for research, theory, and clinical work are considered and concrete recommendations for future directions are provided. While recognizing limitations of existing literature (e.g., homogenous samples, variable methodologies), we conclude that ERP methods represent a promising approach for precision medicine care of patients with ADHD.
Keywords: ADHD, pediatric, ERP, pharmacological treatment, biomarker
1. Introduction
Attention-Deficit/Hyperactivity Disorder (ADHD) is a neurodevelopmental disorder that affects about 7% of children under the age of 18 years (Thomas et al., 2015). Children diagnosed with ADHD present with inattention, hyperactivity, and/or impulsivity (American Psychiatric Association, 2013). This clinical population often experiences functional impairment across several domains (e.g., academic, occupational, social) as well as elevated risk for anxiety, depression, and substance abuse in later life (Brown et al., 2001; Sibley et al., 2010; Sobanski et al., 2007). In 2017, the estimated cost to society of childhood and adolescent ADHD in the United States exceeded 124 billion dollars (Zhao et al., 2019). These data highlight the need to develop effective treatments for children and adolescents diagnosed with ADHD.
There are several evidence-based treatments for ADHD, including pharmacotherapy (e.g., stimulant medication) and behavior therapy (Hoza et al., 2008). A hallmark study on treatments for children with ADHD found that pharmacotherapy alone showed comparable improvements in core ADHD symptoms as the combined treatment of pharmacotherapy and behavior therapy, while both treatment arms outperformed behavior therapy alone (MTA Group, 2004). Stimulant medications, including methylphenidate and amphetamines derivatives, are the most commonly prescribed pharmacological interventions for ADHD (Burcu et al., 2016) and function by increasing pre-synaptic levels of dopamine and norepinephrine in the brain. Positive effects of stimulant medications are reported in a majority (i.e., 60–75%) of children who receive this treatment (Stein et al., 2003; Swanson et al., 1993). However, these statistics are based on group averages and highlight the existence of a subgroup of patients for whom stimulant medications do not significantly reduce ADHD symptoms.
Individual differences in stimulant response may, at least partially, be accounted for by the neurobiological heterogeneity underlying ADHD (Arns and Olbrich, 2014). Consequently, in the past two decades, there has been an increased focus on identifying biomarkers that can inform precision medicine care (i.e., identifying the right treatment for the right person at the right time) for individuals with ADHD (Arns, 2012). Clinical decision-making could, for example, be guided by biomarkers or endophenotypes using ‘pharmaco-electroencephalography’ (EEG; Konopka and Zimmerman, 2014). In particular, the amplitude and latency of EEG-acquired event-related potentials (ERPs) have been discussed as promising biomarkers in research on pharmacological treatments for children diagnosed with ADHD (Konopka and Zimmerman, 2014; Luck, 2014). ERPs could be used in “preclinical research to define potential treatment targets” (Luck et al., 2011, p. 29). Notable benefits of EEG as a biomarker measurement tool include the fact that it is non-invasive and low-cost.
The aim of this review is to summarize the literature on ERP correlates of stimulant response in children diagnosed with ADHD and evaluate the potential for ERP metrics to inform precision medicine care in this population. We start by providing a basic overview of EEG and ERP methods and summarize how these have been applied to research on ADHD. Next, we present what is known about ERP correlates of stimulant response in children with ADHD, with a focus on early sensory processing (i.e., N1, P1, P2) and later attentional processing (i.e., P3a and P3b) components. We conclude with a discussion of major themes in the extant literature, implications for neuroscience theory, research, and clinical practice. We also identify limitations and strengths of the existing research and provide concrete recommendations for future work.
1.1. Electroencephalography Methodology
EEG is a noninvasive, cost-effective measurement technique that captures electrical fluctuations in the cortex with high temporal precision (Nunez and Srinivasan, 2006). Specifically, electrical field activity within the brain is measured as ions move across cell membranes (Niedermeyer and da Silva, 2005). EEG methods have been used to examine cortical brain activity within ADHD populations since the late 1930s (Lenartowicz and Loo, 2014); results from such EEG research consistently demonstrate neural oscillatory differences in individuals diagnosed with ADHD (Loo and Makeig, 2012). Although some research has suggested the potential use of spectral EEG to identify biomarkers of ADHD (e.g., theta/beta ratio; Lubar, 1991), heterogeneity in the disorder and in control samples has hindered meaningful progress in this area (Clarke et al., 2001; Loo et al., 2018).
ERPs are stimulus-locked electric potentials captured through EEG (Woodman, 2010; see Figure 1). ERPs reflect cortical responses to external stimuli or cognitive, sensory, or motor events (Blackwood and Muir, 1990). Components of the ERP are characterized by their relative latencies and positive or negative polarity, denoted by a P (positive) or N (negative), respectively (Woodman, 2010). ERP components are often categorized as relatively “early” and “late” processes, although most components of interest peak within the first 500 milliseconds following stimulus presentation (M. J. Taylor and Baldeweg, 2002). Early components are believed to reflect neuronal sensory processing and categorization and include P1, N1, P2 and N2 components (Woodman, 2010). Later components are believed to reflect cognitive attention and executive control processes and include the P3a and P3b components, or if measured through latency, the P300 waveform (Woodman, 2010). This review will focus on both early and late processing components and the extent to which their amplitudes (i.e., magnitude of positive or negative polarity) and latencies (timing of peak amplitude) are impacted by stimulant medications. Whenever possible, we will also consider whether stimulant-associated ERP changes correspond to behavioral improvements.
Figure 1.
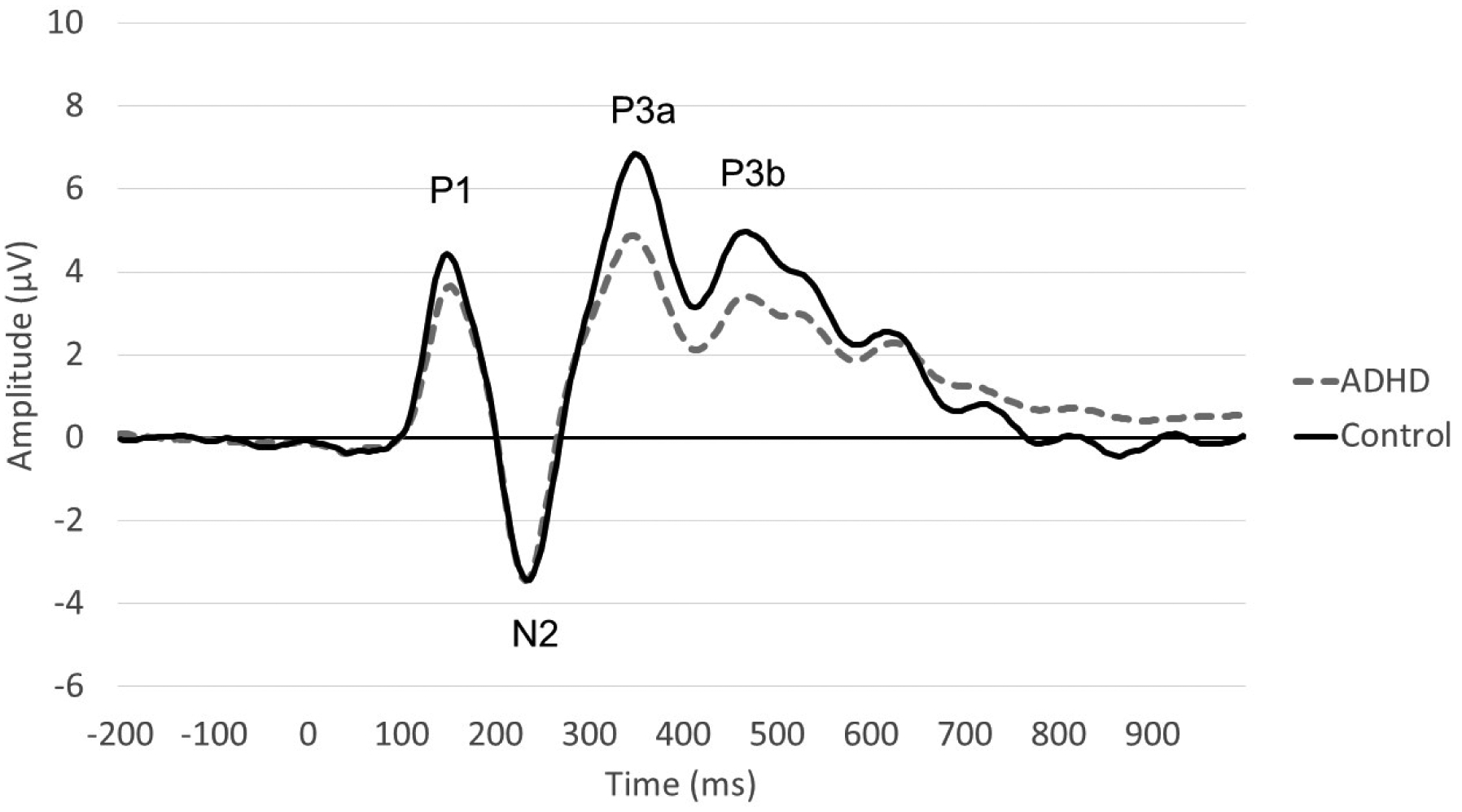
Event-Related Potential (ERP) Waveform
Both auditory and visual performance tasks elicit ERPs. One of the most common auditory paradigms is the auditory oddball task (Squires et al., 1975), in which the participant is presented with repetitive auditory stimuli, with a deviant auditory stimulus randomly interspersed. Visual paradigms include visual oddball tasks, working memory tasks, the Stroop task (Stroop, 1935), the Go/No Go task (GNG Donders, 1969), and variations of the continuous performance task (CPT; for additional review on variations see Riccio et al., 2002). Working memory tasks include lexical or pictorial stimuli n-backs. Each of these tasks targets unique neurocognitive processes, such as inhibitory control or novelty detection, as well as shared processes, such as attention maintenance and executive control. As such, all tasks elicit similar ERP waveforms with early components reflecting visual and auditory recognition and later components reflecting action preparation, task execution, and attention maintenance.
ERP differences among individuals with ADHD have been examined extensively. A recent meta-analysis of 52 articles including children and adults with ADHD (n = 1576) and without ADHD (n = 1794) found a moderate effect size for shorter Go-P100 latencies among individuals diagnosed with ADHD relative to non-ADHD participants (d = −0.33; Kaiser et al., 2020). In contrast to early processing, stronger group differences emerged for later ERP components (Kaiser et al., 2020). Specifically, ADHD was associated with smaller cue-P300 amplitudes, longer Go-P300 latencies, smaller NoGo-P300-amplitudes, and longer NoGo-P300 latencies (absolute d range = .35 – .56; Kaiser et al., 2020). These results are consistent with the hypothesis that neurocognitive deficits in ADHD are most apparent during action-execution attention processing phases, as opposed to basic sensory processing and categorization. Thus, in the current review we expected that stimulant effects would likewise be greatest for the P300 as compared to earlier ERP components.
2. Methods
To identify studies relevant to this review, we conducted a Boolean search of multiple databases (PsychINFO, PubMed, Science Direct, Google Scholar), using keywords “ADHD, EEG, ERP, stimulants, pediatric, child” with operators “and” and “or.” Additionally, we cross-referenced the bibliographies of identified studies for additional publications. Inclusion criteria were publication in the English language, pediatric sample (<18 years), administration of stimulant medications, and investigation of ERP components. Publication year was not considered as an inclusion/exclusion criterion. We focused on stimulus-locked components rather than on response-locked components (for example, we excluded articles, such as Groom et al., 2013). Because very few studies reported on ERP components with peak latencies after that of the P300 (e.g., late positive potential), such later-occurring components are not included in this review1. Forty-three studies met inclusion criteria and are summarized in Table 1.
Table 1.
Overview of Available Studies
| Author(s) | Year | Sample Size (%male) | Age (years) | Diagnostic Criteria | Medication | Study Design | ERP Task | ERP component | Component window (ms) | Effect of Medications | Behavioral Changes |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Aasen et al. | 2018 | 57 (70%) | 7–17 | DSM-IV | MPH | Repeated measures | Cued visual go/no-go task | Early P3 | 378 | ↓amplitude | Enhanced task performance |
| Late P3 | 428 | No change | |||||||||
| Broyd et al. | 2005 | 36 (100%) | 8–14 | DSM-IV | MPH | Repeated measures | Cued auditory go/no-go task | N1 | 70–150 | ↓latency | Enhanced task performance |
| P2 | 150–250 | ↓latency | |||||||||
| N2 | 170–290 | ↓latency | |||||||||
| P3 | 250–500 | ↓amplitude | |||||||||
| Coons et al. | 1987 | 19 (NR) | 6–12 | Ratings exceeded 2 SD on Home Activities Scale (Parent) and Conners Teachers Questionnaire | MPH | Placebo/MPH (blind) | Sternberg task (visual) | P3b | NR | No change | Enhanced task performance |
| Visual CPT task | P3b | NR | ↓latency | Enhanced task performance | |||||||
| Dolu et al. | 2019 | 36 (78%) | 7–12 | DSM-IV | MPH | Repeated measures | Auditory Oddball task | P3 | NR | ↑amplitude | Decrease in parent ratings of ADHD symptoms |
| Fitzpatrick et al. | 1992 | 19 (NR) | 6.9–11.5 | DSM-III | MPH | Repeated measures (placebo) | Visual CPT task | P3b | NR | ↑amplitude Latency: no change | Enhanced task performance; decrease in parent and teacher ratings of ADHD symptoms |
| Frank | 1993 | 68 (100%) | 7–12 | DSM-III-R | MPH | Repeated measures (placebo) | Visual ERP test | P3 | 280–380 | No change | - |
| Hermens et al. | 2005 | 68 (82%) | 11–17 | DSM-IV | MPH | Repeated measures | Auditory oddball task | N1 | 70–140 | No change | Enhanced task performance |
| P2 | 210–220 | No change | |||||||||
| N2 | 140–300 | No change | |||||||||
| P3 | 220–550 | ↑amplitude; ↑latency | |||||||||
| Janssen et al. | 2016 | 81 (75%) | 7–13 | DSM-IV | MPH | Repeated measures | Stop Signal task | N2 | 215–265 | ↑amplitude | Enhanced task participants who showed ERP change |
| P3 | 300–400 | ↑amplitude | |||||||||
| Jonkman et al. | 1997 | 18 (89%) | 7–13 | DSM-III-R | MPH | Repeated measures | Auditory Oddball task | N1 | 50–150 | No change | Enhanced performance on visual task; no change in auditory task |
| N2 | 200–290 | No change | |||||||||
| P2 | 110–250 | No change | |||||||||
| P3b | 400–800 | ↑amplitude | |||||||||
| Visual Oddball task | N1 | 50–150 | No change | ||||||||
| P1 | 50–150 | No change | |||||||||
| N2 | 250–450 | ↑amplitude | |||||||||
| P2 | 175–230 | No change | |||||||||
| P3 | 275–400 | No change | |||||||||
| P3b | 400–800 | ↑amplitude | |||||||||
| Jonkman et al. | 1999 | 14 (93%) | 7–13 | DSM-III-R | MPH | Repeated measures (placebo) | Eriksen Flanker task (visual) | P2 | 160–300 | No change | Enhanced task performance |
| N2 | 290–450 | No change | |||||||||
| P3 | 400–800 | No change | |||||||||
| Jonkman et al. | 2000 | 14 (93%) | 7–13 | DMS-III-R | MPH | Repeated measures (placebo) | Irrelevant probe technique (visual) | N1 | NR | No change | Enhanced task performance |
| P1 | NR | No change | |||||||||
| P2 | NR | No change | |||||||||
| NC | NR | No change | |||||||||
| P3 | NR | ↑amplitude | |||||||||
| Keage et al. | 2008 | 439 (79%) | 6–17 | DSM-IV-TR | MPH, Dexampheta mine | Repeated measures | One-back working memory task (visual) | N1 | NR | No change | Enhanced task performance |
| P150 | NR | No change | |||||||||
| N300 | NR | No change | |||||||||
| Klorman et al. | 1983 | 27 (70%) | NR | Ratings exceeded 2 SD on two scales | MPH | Repeated measures (placebo) | Oddball task | N140 (auditory) | NR | ↑amplitude | Enhanced performance |
| Klorman et al. | 1988 | 63 (NR) | 6–12 | Ratings exceeded 2 SD on two scales | MPH | Repeated measures | CPT task | P3b | NR | ↑amplitude | Decrease in parent and teacher ratings of ADHD symptoms |
| Klorman et al. | 1990 | 53 (92%) | 6–12 | Ratings exceeded 2 SD on two scales | MPH | Repeated measures (placebo) | Multimodal Oddball | N1 auditory | 120-P2 | ↑amplitude | Enhanced performance |
| N1 visual | 120-P2 | No change | |||||||||
| Klorman et al. | 1992 | 48 (88%) | 12–18 | DSM-III | MPH | Repeated measures (placebo) | Sternberg task (visual) | P3 | 280–700 | ↑amplitude | Enhanced performance |
| Klorman et al. | 1994 | 107 (85%) | 5.6–11.9 | Ratings exceeded 1.5 SD | MPH | Repeated measures (placebo) | Sternberg task (visual) | P3b | NR | ↑amplitude and latency | Enhanced performance; decrease in parent and teacher ratings of ADHD symptoms |
| Lawrence et al. | 2005 | 36 (100%) | 8–13 | DSM-IV | MPH | Repeated measures | CPT | N1 | 100–190 | ↑amplitude (normalized to controls) | Enhanced performance; no change in RT |
| Auditory go/no-go task | P2 | 190–250 | No change | ||||||||
| N2 | 200–300 | No change | |||||||||
| P3 | 300–450 | No change | |||||||||
| Lopez et al. | 2004 | 11 (91%) | 9–14 | DSM-IV | D-Amphetamine | Repeated measures | Visual Oddball task; Stroop task | P3 | NR | ↑amplitude | Increased performance on Stroop task |
| Lubar & Swartwood | 1995 | 35 (100%) | 9–11 | DSM-III-R | MPH | Between group comparison (TD, ADHD+meds; ADHD-meds) | Auditory Oddball | N1 | NR | No change in habituation | - |
| N2 | NR | No change in habituation | |||||||||
| P2 | NR | No change in habituation | |||||||||
| P3 | NR | No change in habituation | |||||||||
| Michael et al. | 1981 | 50 (86%) | 5.9–13 | Ratings exceeded 2 SD on two scales | MPH | Repeated measures | Visual CPT | P3 | 200–600 | ↑amplitude | Enhanced performance |
| Ogrim & Hestad | 2013 | 29 (62%) | 7–16 | DSM-IV | MPH, D-Amphetamine | Random assignment to two treatment groups (medication; neurofeedback) | Visual go/no-go task | P3 | 300–500 | 8 of 12 REs showed ↑amplitude | - |
| Ogrim et al. | 2014 | 98 (67%) | 7–17 | DSM-IV | MPH | Grouped as REs and non-REs | Visual go/no-go task | IC cue P3 | 220–400 | REs > non-REs: amplitude | - |
| IC P3 no-go | 250–470 | REs > non-REs: amplitude | |||||||||
| Ogrim et al. | 2016 | 87 (69%) | 8–17 | DSM-IV | MPH | Grouped as REs and non-REs | Visual go/no-go task | Cue P3 | 270–370 | No change | REs had greater enhanced performance than non-REs |
| P3go | 260–400 | No change | |||||||||
| N2 no-go | 220–330 | No change | |||||||||
| P3 no-go | 300–500 | REs > non-REs: ↑amplitude | |||||||||
| Ogrim & Kropotov | 2019 | 87 (69%) | 8–17 | DSM-IV | MPH | Comparison pre meds vs. post meds | Visual go/no-go task | P1 | 100–220 | No change | Differences in omissions and RT variability when comparing no meds to meds |
| Cue-P3 | 270–370 | No change | |||||||||
| P3 go | 260–400 | No change | |||||||||
| N2 no-go | 220–330 | No change | |||||||||
| P3 no-go | 300–500 | REs > non-REs: ↑amplitude | |||||||||
| N1 auditory | 100–170 | No change | |||||||||
| P2 auditory | 170–250 | No change | |||||||||
| Grouped as REs and non-REs | Visual go/no-go task | P1 | 100–220 | Differences in reaction time variability between REs and non-REs | |||||||
| Cue-P3 | 270–370 | ||||||||||
| P3 go | 260–400 | ||||||||||
| N2 no-go | 220–330 | ||||||||||
| P3 no-go | 300–500 | ||||||||||
| N1 auditory | 100–170 | ||||||||||
| P2 auditory | 170–250 | ||||||||||
| Okazaki et al. | 2002 | 19 (100%) | Mean 11 | DSM-IV | MPH | Repeated measures | Visual CPT-AX | P2 | 250 | ↑amplitude (normalized to controls); ↑latency | Enhanced performance |
| P3 | NR | Altered scalp distribution | |||||||||
| Ozdag et al. | 2004 | 46 (100%) | 7–14 | DSM-IV | MPH | Repeated measures | Auditory Oddball task | P1 | NR | ↑amplitude | - |
| N2 | NR | ↓latency | |||||||||
| P3 | NR | ↓latency; ↑amplitude | |||||||||
| Paul-Jordanov et al. | 2010 | 27 (89%) | 12.0–12.8 | DSM-IV | MPH | Repeated measures | Visual go/no-go | P3 | 260–328 | ↑amplitude | Enhanced performance |
| P3 go/no-go difference | ↑amplitude | ||||||||||
| Pliszka et al. | 2007 | 12 (75%) | 9–15 | DSM-IV | MPH | Repeated measures (placebo) | Visual Stop Signal Task | Early no-go N200 | 120–170 | ↑amplitude | Enhanced performance |
| No-go N200 | 170–220 | ↑amplitude | |||||||||
| Early no-go P300 | 300–400 | ↑amplitude | |||||||||
| Late no-go P300 | 400–500 | ↑amplitude | |||||||||
| Prichep et al. | 1976 | 24 (100%) | 8–11 | Obtaining a mean score of 1.5 on the hyperactivity factor on the Conner’s Teacher Rating Scale | MPH | Repeated measures (placebo) | Single/double click guessing paradigm | P50 | NR | No change | |
| N100 | NR | No change | |||||||||
| P200 | NR | ↑amplitude | |||||||||
| N250 | NR | ↓amplitude | |||||||||
| P300 | NR | No change | |||||||||
| Rubinson et al. | 2019 | 37 (54%) | 9–17 | DSM-5 | MPH | Repeated measures (placebo) | Visual go/no-go task | P3 | 250–400 | ↑amplitude | Enhanced performance |
| Sangal & Sangal | 2004 | 20 (60%) | 6–12 | DSM-IV | MPH-XR | Grouped as REs and non-REs | Auditory Oddball Task | P3 | 320–480 | REs> non-REs: frontal:parietal amplitude | Significant differences between REs and non-REs in ADHD rating scores |
| Visual Oddball Task | P3 | 320–480 | REs = non-REs | Significant differences between REs and non-REs in ADHD rating scores | |||||||
| Sangal & Sangal | 2006 | 58 (72%) | 6–14 | DSM-IV | MPH, Atomoxetine | Repeated measures | Auditory and visual oddball | P3 auditory | 320–480 | REs > non-REs: pre-treatment amplitude | |
| Satterfield et al. | 1972 | 52 (NR) | 6–9 | 6 (or more) ADHD symptoms | MPH | Repeated measures (placebo); grouped as REs and non-REs | Auditory task | P2 | 180 | REs > non-REs: peak-to-peak | |
| N2 | 280 | amplitude (P2-N2) | |||||||||
| Seifert et al. | 2003 | 37 (100%) | 7.2–11.7 | ICD-10 | MPH | Repeated measures | Visual CPT-OX | P3a | 257–408 | ↑amplitude (normalized to controls) | |
| Smithee et al. | 1998 | 26 (77%) | 6.5–12 | DSM-IV | MPH | Repeated measures (placebo) | Visual discrimination task | P3b | 450–520 | No change | Enhanced task performance; decrease in parent and teacher ratings of ADHD symptoms |
| Sunohara et al. | 1999 | 40 (80%) | 10–12 | Visual CPT | MPH (low and high dose) | Repeated measures (placebo) | Visual CPT | N2 | NR | ↑latency at high dose | Enhanced performance at low and high dose MPH |
| P2 | NR | ↑latency at high dose | |||||||||
| P3 | NR | ↓latency at low and high dose | |||||||||
| Taylor et al. | 1993 | 64 (78%) | 7–12 | DSM-III-R | MPH | Repeated measures (placebo) | Visual oddball | P3a | NR | ↓latency (normalized to controls) | No change |
| P3b | NR | ↓latency (normalized to controls) | |||||||||
| Verbaten et al. | 1994 | 12 (83%) | Mean 11.2 | DSM-III-R | MPH | Repeated measures | Visual CPT-X | N1 | 50–200 | No change | Enhanced performance |
| P2 | 120–250 | No change | |||||||||
| N2 | 250–400 | ↑amplitude | |||||||||
| P3b | 400–700 | ↑amplitude | |||||||||
| Williams et al. | 2008 | 102 (100%) | 8–17 | DSM-IV | MPH | Repeated measures | Facial emotion recognition task | P120 | 80–150 | ↑amplitude | Improved ratings for emotional instability and |
| P300 | 300–400 | ↓latency | hyperactivity | ||||||||
| Winsberg et al. | 1997 | 28 (NR) | 7–12 | DSM-III-R | MPH | Repeated measures (placebo) | Auditory oddball | N1 | 50–150 | No change | Enhanced performance; Improved ADHD symptom ratings |
| N2 | 150–250 | No change | |||||||||
| P2 | 150–250 | No change | |||||||||
| P3 | 250–550 | ↑amplitude | |||||||||
| Zillessen et al. | 2001 | 17 (100%) | 7–11 | ICD-10 | MPH | Repeated measures | Visual CPT (modified) | P1 | 51–164 | No change | - |
| P2 | 164–257 | No change | |||||||||
| P3a | 257–408 | ↑amplitude | |||||||||
| P3b | 408–527 | No change |
Note. ERP=Event related potential; DSM=Diagnostical and Statistical Manual of Mental Disorders; ICD-10=International Classification of Diseases, 10th Edition; ADHD=Attention-Deficit/Hyperactivity Disorder; MPH=Methylphenidate (immediate release); MPH-XR=Methylphenidate extended release; REs=responders; non-REs=non-responders; CPT=continuous performance task; TD=typically developing participants; NR=not reported; we omit results from ERP components that occur after the P300 (e.g., CNV). Component labels are reported as published
Although a wide range of study designs has been used, a large portion of studies employed a “repeated measures” approach, whereby participants completed ERP tasks and behavioral questionnaires before and after administration of stimulant medications (see Aasen et al., 2018 as an example for this approach). A second, commonly used study design included a baseline assessment followed by a random assignment to either stimulant medication or placebo treatment condition (Jonkman et al., 2000; Rubinson et al., 2019). Some studies included a typically developing (TD) comparison group and specifically examined whether stimulant medications would “normalize” ERP profiles of children with ADHD such that they did not differ from TDs (for example, see Lubar et al., 1995). Finally, several studies contrasted the ERP profiles of ADHD patients who were categorized as stimulant medication “responders” (REs) or “non-responders” (non-REs) according to whether clinically significant behavioral change was or was not observed, respectively. Regarding stimulant class, most studies in this review examined the effects of methylphenidate (MPH), but amphetamines were also investigated (see Table 1). One study (Sangal et al., 1995) that examined the impact of pemoline was excluded from the review due to pemoline’s limited current use. Although a majority of studies focused on middle childhood (7–12 years), some investigations included adolescents as well (see Table 1). The following sections synthesize results across the 42 studies and present findings organized by early and late sensory processing components.
3. Results
3.1. Early Sensory Processing
3.1.1. Sensory ERP Components
Within the first 300 milliseconds (ms) after stimulus onset, a series of early ERP components are visible in the ERP waveform. Typically, these include a temporal order of the P1 (100 ms), the N1 (150 ms), P2 (200 ms), and N2 (250 ms). The P1 is evoked via visual stimuli and is associated with basic sensory processing in the visual field (Hillyard and Anllo-Vento, 1998). The N1 wave consists of a negatively valanced amplitude and is thought to indicate information extraction from presented stimuli (Näätänen and Picton, 1987). The N1 component is most pronounced when unexpected or unpredictable stimuli are presented (Sur and Sinha, 2009) and is associated with selective attention (Mueller et al., 2008). The subsequent P2 wave is a positively valanced amplitude, which is associated with higher-order cognitive processes (e.g., recognizing of stimulus features, semantic processing) as well as inhibition of competing information (Luck and Hillyard, 1994). The N2 wave is associated with encoding stimulus changes and stimulus discrimination (Sur and Sinha, 2009).
3.1.2. Sensory ERP Components and ADHD
Several studies have examined potential group differences in sensory ERP components between ADHD patients and control participants. Results suggest that children and adults with ADHD demonstrate reduced P1 amplitude during attention tasks (Banaschewski et al., 2003; Barry et al., 2009; Papp et al., 2020). However, only P1 latency was significantly different among pooled samples (Kaiser et al., 2020). At least one study has reported attenuated N1 amplitudes in young ADHD participants (Barry et al., 2003); a study of adolescents failed to find this difference (Johnstone, 2001). Investigations of P2 and N2 likewise report inconsistent results, including greater amplitude, longer latency, reduced latency, or no differences among youth with ADHD (for review see Barry et al., 2003). Critically, early ERP components appear to be moderated by individual characteristics, including cognitive abilities and medication status (Kaiser et al., 2020). These moderator interactions underscore the heterogeneity of the ADHD population and likely explain mixed findings for associations between ADHD and early ERP components. In sum, individuals diagnosed with ADHD appear to differ in some aspects from non-ADHD individuals in sensory processing; however, results are notably inconsistent.
3.1.3. Stimulant Effects on Sensory ERP Components
Review of the extant literature indicates that stimulant effects on early sensory processing components are small or negligible (see Table 1 for detailed results). However, a minority of studies report significant differences following stimulant medication administration, particularly for early negative components (i.e., N1, N2). For example, two studies reported increased amplitude of the N1 (Klorman et al., 1992; Lawrence et al., 2005) and one study reported decreased N1 latency (Broyd et al., 2005) following stimulant treatment. Likewise, four studies reported increased N2 amplitude following MPH (Janssen et al., 2016; Jonkman et al., 1997; Pliszka et al., 2007; Verbaten et al., 1994), while a fourth study found decreased amplitude specifically for the N250 component (Prichep et al., 1976). MPH was associated with significantly shorter N2 latency in two studies (Broyd et al., 2005; Ozdag et al., 2004), but longer latency in a third (Sunohara et al., 1999); the latter study differed in that the latency effect was only found at high MPH doses (Sunohara et al., 1999). Of note, and as outlined in Table 1, a considerable number of studies reported that stimulant medications did not significantly alter the profiles of N1 or N2 components.
Differences among positively-valanced early components (i.e., P1, P2) are less commonly examined and reported. An exception to this is an investigation by Broyd and colleagues (Broyd et al., 2005), who found that MPH was associated with decreased latency across all early processing components among male youth with ADHD during a cued auditory go/no-go task. Another investigation by Ozdag and colleagues (Ozdag et al., 2004) likewise reported decreased N2 latency following MPH administration among a sample of school-age males, suggesting the finding may be specific to this demographic. The minimal findings of associations between stimulant medication and positively-valanced early components aligns with few reported differences in P2 amplitude or latency between ADHD patients and controls (as described above). However, given that a recent meta-analysis had reported group differences in ADHD versus control participants in the P1 latency (Kaiser et al., 2020), the minimal effect of stimulants on the P1 was somewhat surprising.
As described earlier, individual differences in cognition and medication history may moderate stimulant effects on early ERP components in ADHD. An additional potential moderator is medication tolerance, i.e. side effects. Ogrim and Kropotov (2019) reported that individuals who experienced stimulant side effects such as insomnia, loss of appetite, irritability, and anxiety had increased N1 and P2 amplitudes (Ogrim and Kropotov, 2019). Another possibility is that developmental changes in early processing components (e.g., Broyd et al., 2005) attenuate the overall effects. In fact, few studies reviewed herein include age as a potential moderator variable (see Broyd et al., 2005 as a notable exception). Overall, the literature investigating early sensory components tentatively suggests a possible effect of stimulant medications on the amplitude of negative components.
Behavioral correlates of early ERP differences associated with MPH are limited. Broyd and colleagues (Broyd et al., 2005) investigation found that MPH was associated with reduced commission (but not omission) errors during a Go/NoGo task, suggesting that MPH specifically improves inhibitory motor control among children with ADHD. Another study reported that MPH was associated with improved stop signal task performance among children with ADHD, and this was also associated with increased N200 amplitude (Pliszka et al., 2007). In contrast, Janssen and colleagues (Janssen et al., 2016) attributed increased N2 amplitude during the stop signal task to developmental and/or practice effects, rather than MPH.
3.2. Later Attention Processes
3.2.1. Attention ERP Components
There are multiple ERP sub-components in the time range of the P300 wave (see Polich, 1986 for a detailed review). A key distinction can be made between the P3a, which is typically maximal at frontal scalp electrodes, and the P3b, which is maximal at the parietal scalp region (Squires et al., 1975). The P3a has been associated with novelty detection, while the P3b reflects preparation and execution (Polich, 2012; Polich and Kok, 1995). Several terms are used interchangeably for the P3b component, including P3 and P300 (Luck, 2014). Although these components have been studied extensively, there “is no clear consensus about what neural or cognitive processes are reflected by the P3 wave” (Luck, 2014). Research has, however, uncovered factors that influence both amplitude and latency of the P300. For example, the P300 amplitude is larger when participants allocate greater resources toward a task (Israel et al., 1980; Wickens et al., 1983). The amplitude also increases as the probability of the stimulus (i.e., task relevant and novel distracter) decreases (e.g., Duncan-Johnson and Donchin, 1977). The latency of the P300 appears to be a function of stimulus classification speed (e.g., Duncan-Johnson, 1981; Polich, 1986).
3.2.2. Attention ERP Components and ADHD
Children diagnosed with ADHD have been shown to have P3a and P300 features that differ significantly from those of healthy control participants. For example, the P3a amplitude has been shown to be significantly smaller in ADHD patients relative to TD controls (e.g., Gumenyuk et al., 2005; Liotti et al., 2005). Further, decreased P300 amplitude has been reported during selective attention tasks in ADHD participants compared to control participants (e.g., Jonkman et al., 1997; Loiselle et al., 1980). Similarly, attenuated P300 amplitude following target stimuli in oddball tasks has been associated with ADHD (Jonkman et al., 1997; Satterfield, et al., 1972; Satterfield et al., 1990). In contrast, findings regarding peak latencies of the P300 in children diagnosed with ADHD are mixed (see Barry et al., 2003 for a detailed review). A recent meta-analysis of 52 articles concluded that group differences between ADHD and control participants were most robust for later ERPs (Kaiser et al., 2020). For example, the ADHD group was found to have smaller cue-P300-amplitudes, longer Go-P300-latencies, and smaller NoGo-P300-amplitudes relative to the non-ADHD group (Kaiser et al., 2020). Importantly, this meta-analysis included children and adults, the latter being a distinguishing feature from this review.
3.2.3. Stimulant Effects on Attention ERP Components
A majority of studies (n = 39) included in the current review examined the association between stimulant response and aspects of the P300 waveform (i.e., P3a or P3b), highlighting this component’s relevance to the ADHD literature. A minority of studies focused specifically on the P3a, as opposed to the P3b or broader P300 component. These reports broadly indicated an increase in P3a amplitude following stimulant medication administration. Specifically, three studies included in this review (Pliszka et al., 2007; Seifert et al., 2003; Zillessen et al., 2001) reported an increase in P3a waveform in response to stimulants. In contrast, two studies reported no change (Jonkman et al., 1997; Taylor et al., 1993) and one study reported a decrease in P3a amplitude (Aasen et al., 2018). The inconsistencies in findings do not seem attributable to participant age or modality. In fact, all six studies used visual paradigms. However, differences could be attributable to study task as increase in P3a amplitude was found for studies using the CPT task (Seifert et al., 2003; Zillessen et al., 2001), no change was found for studies using the Oddball task (Jonkman et al., 1997; Taylor et al., 1993), and a decrease was found for the Go-No/Go task (Aasen et al., 2018).
Similar to the P3a, results for the P3b generally suggest an increase in amplitude following stimulant medication administration. Specifically, 17 studies reported that, within a sample of children diagnosed with ADHD, the amplitude of the P300 increased following administration of stimulant medication relative to a placebo condition or no medication (Dolu et al., 2019; Fitzpatrick et al., 1992; Hermens et al., 2005; Janssen et al., 2016; Jonkman et al., 2000; Jonkman et al., 1997; Klorman et al., 1992; Klorman et al., 1994; Klorman et al., 1988; Lopez et al., 2004; Michael et al., 1981; Ozdag et al., 2004; Paul-Jordanov et al., 2010; Pliszka et al., 2007; Rubinson et al., 2019; Verbaten et al., 1994; Winsberg et al., 1997). Three studies in which ADHD patients were compared to controls reported that P300 amplitudes among participants diagnosed with ADHD were normalized with stimulant administration (Seifert et al., 2003; Sunohara et al., 1999; Taylor et al., 1993). In contrast, eight studies reported no change in P300 amplitude after stimulants were administered (Aasen et al., 2018; Coons et al., 1987; Frank, 1993; Jonkman et al., 1999; Lawrence et al., 2005; Prichep et al., 1976; Smithee et al., 1998; Verbaten et al., 1994). There was no clear pattern of participant age, task type, or modality (i.e., auditory versus visual) distinguishing studies with significant versus null results. Finally, one study reported a decrease in P300 amplitude following stimulant medication administration, despite improved task performance (Broyd et al., 2005). This study was unique in that it was the only study reviewed to use a cued Go-No/Go task, suggesting that introduction of a preparatory stimulus (i.e., cue) may change the neurocognitive function of the P300 component.
Additional insight was provided by studies that classified ADHD patients as responders (REs) or nonresponders (non-REs) to stimulant medications and examined group differences in P300 amplitude. Ogrim and colleagues (Ogrim et al., 2014) found that, among a sample of 98 youth (ages 7–17) with ADHD, behavioral response to stimulants was associated with normal cue P3 and attenuated no-go P3 amplitudes pre-medication. Using a sample of 87 ADHD patients (ages 8–17) this same group found that REs and non-REs differed in medication-induced changes in several ERP components, such as the no-go P3, cue-P3, and CNV, and in baseline (i.e., unmedicated) amplitude of the no-go P3 (Ogrim and Kropotov, 2019). Sangal and colleagues (Sangal et al., 2004) reported that topographic distribution of the auditory P300 predicted behavioral response to stimulant medication among youth with ADHD. Specifically, they found that enhanced P300 at frontal versus parietal was associated with RE versus non-RE categorization. In contrast, topography of a visual P300 was not predictive of RE status, and neither auditory nor visual mean P300 amplitudes or latencies was associated with pemoline response.
In contrast to the P300 amplitude, stimulant effects on the P300 latency appear to be both less studied and less consistent. Four studies (Coons et al., 1987; Ozdag et al., 2004; Sunohara et al., 1999; Williams et al., 2008) reported a decrease in P300 latency following stimulant administration; three studies (Fitzpatrick et al., 1992; Sangal and Sangal, 2004; Sangal et al., 1995) reported no change in P300 latency following stimulant administration; and two studies (Hermens et al., 2005; Klorman et al., 1994) reported an increase in latency associated with stimulant administration. Focusing on studies that grouped participants into REs and non-REs, Sangal and Sangal (2006) was the only study to report a smaller mean latency value for REs compared to non-REs.
It is noteworthy that the studies reviewed here used a wide range of task designs with variable cognitive load, which likely moderated the results. Specifically, studies that reported on the P300 component (n = 39) used visual and auditory Oddball (n =14), Go/no-go (n = 10), CPT (n = 10), Sternberg memory (n = 3), and Stop Signal (n = 2) tasks, in addition to several others (e.g., Eriksen Flanker task, Stroop task, Irrelevant Probe Technique; see Table 1 for more details)2. Changes in P300 amplitude and latency as a function of stimulant medication were reported across all task types, suggesting that stimulant medications consistently alter ERP profiles in tasks requiring a wide range of cognitive functions (e.g., attention, inhibitory control, memory).
Another question of interest is whether changes in the P300 component coincide with changes at the behavioral level. Studies included in this review examined participants’ performance on study tasks as well as changes in ADHD symptoms. The overwhelming majority of studies examining the P300 (~65%) reported that participants’ performance on cognitive tasks improved with stimulant medication (e.g., decreased reaction times; reduction in commission and omission errors). For example, Lopez and colleagues (Lopez et al., 2004) reported that, relative to a pre-medication baseline assessment, participants diagnosed with ADHD showed increased P3 amplitude and greater inhibitory control during the Stroop task following administration of dextroamphetamine. Other studies measured changes in ADHD symptoms as primary outcomes. These studies likewise reported increased P300 amplitudes in addition to reduced severity of parent- and/or teacher-rated ADHD symptoms with administration of stimulants (e.g., Klorman et al., 1988; Klorman et al., 1994; Winsberg et al., 1997).
4. Discussion
4.1. Theoretical Implications
When considered together, results from early sensory and later attention processing ERP components inform theoretical models of ADHD. Specifically, differences between ERP profiles among ADHD as compared to TD patients at baseline and following administration of stimulant medications contribute to our understanding of underlying pathophysiology in ADHD. Although some studies reported a significant effect of stimulant medications on early sensory processing components (e.g., N1), the most robust finding across studies occurred in late-processing components. Specifically, stimulants appear to increase the amplitude of the P300 within pediatric patients diagnosed with ADHD. As discussed in foregoing sections, greater P300 amplitude is associated with increased allocation of neural resources toward a task (Israel et al., 1980; Wickens et al., 1983). At the neural level, ERP amplitudes primarily derive from extracellular charges associated with neural depolarization during action potentials (Luck, 2014). Simultaneous firing of local networks of pyramidal cells results in greater ERP amplitude detected at the scalp; therefore, increased P300 amplitude likely reflects greater coordination of cortical network activation, particularly during the action execution phase of attention processing. Thus, stimulant medication-associated changes to P300 morphology suggest that cognitive and behavioral impairments in ADHD are associated with reduced activation and coordinated recruitment of cortical networks that can be mitigated by increasing the availability of pre-synaptic catecholamines.
The neurophysiological research reviewed here aligns with the theoretical framework of Posner’s attention network (Petersen and Posner, 2012; Posner and Petersen, 1990). Specifically, stimulant medications appear to primarily target later-occurring ERPs, which corresponds to the third attention network, as described by Posner and Petersen (Petersen and Posner, 2012; Posner and Petersen, 1990). Given that the third attentional network (“alerting/vigilance”) is said to maintain attention, our results suggest that view that ADHD mostly represents a deficit in the maintenance of attention and cortical arousal. Further, our results align with the theoretical view that the third network is rooted in frontal-striatal cortical networks where catecholamine density is high.
4.2. ERP Markers for Precision Medicine
One of the primary objectives of this review was to inform the precision medicine approach to ADHD treatment, which considers characteristics of each patient (e.g., biomarkers obtained through EEG/ERP methods) to inform clinical decisions (e.g., whether to prescribe medications; selection of medication class). This review identified two ERP components as potential candidates for individualized treatment approaches for pediatric ADHD: the amplitude of the N1 components and the amplitude of the P300 component. As described above, there appear to be greater inconsistencies for early sensory processing (e.g., N1) when compared to later sensory processing (e.g., P300), both as biomarkers of ADHD diagnosis and treatment response. Thus, the P300 component is the most promising ERP biomarker for precision medicine care in ADHD, to date. Specifically, reduced P300 amplitude at baseline is a marker of high risk for ADHD diagnosis, while normalization of P300 amplitude following stimulant administration is a predictor of positive response to this treatment. The latter conclusion follows from our report that, in the majority of studies, enhanced P300 amplitude following stimulant administration also corresponded to improved behavioral response. From this finding, we can hypothesize that the mechanism by which stimulants affect behavior involves fronto-parietal circuitry associated with attention allocation and novelty detection. However, the direction of effect cannot be unequivocally determined without additional study designs, including estimation of dose-dependent effects on neurophysiology and behavior; and manipulation of P300 components using alternative means, such as aerobic exercise (Ludyga et al., 2017).
Among the studies we reviewed, the variability in experimental protocols and study samples precluded identification of a precise threshold for P300 amplitude to predict stimulus response. Rather than continue to evaluate general effects of stimulant medications on the P300 component, we encourage researchers to focus on standardizing experimental protocols across study sites, and to pool their data with the goal of establishing a large, diverse normative dataset. Several additional limitations need to be addressed in order for ERP components to be effectively used as precision medicine biomarkers among clinical populations with ADHD.
Test-retest reliability of ERP measures: The clinical utility of biomarkers for precision medicine is dependent on the consistency of the measure within an individual. Few ERP studies in ADHD have focused on test-retest reliability. Multi-site studies similar to the Autism Biomarkers Consortium for Clinical Trials (Levin et al., 2020; McPartland et al., 2020), will be needed to investigate both reliability and generalizability of potential EEG-based biomarkers.
Age-related changes in ERP measures: ADHD is a developmental disorder with symptoms emerging in childhood during ongoing brain development. Studies identified in this review largely focused on the elementary school age range when stimulant medications are most often used; however, whether these responses are consistent at earlier ages, when the effectiveness of stimulants is also more varied (Barbaresi et al., 2020), remains unclear.
Specificity of EEG biomarkers for individuals with coexisting conditions: The vast majority of children and adolescents with ADHD have coexisting conditions including learning disorders, neurodevelopmental disorders (e.g., autism spectrum disorder), and mental health disorders (e.g., anxiety, depression). Precision medicine is most needed to aide medication management of complex cases of ADHD where identifying effective medications and balancing side effects is often challenging (Barbaresi et al., 2020). However, few EEG or ERP studies in ADHD have examined similarities and differences in ERP measures in children with ADHD and commonly occurring coexisting conditions.
Predicting side effects: Adverse side effects (Graham and Coghill, 2008; e.g., reduced appetite, sleep challenges, irritable mood, behavioral withdrawal), can lead to discontinuation of a stimulant medication, despite a positive response in ADHD symptoms. This is especially true in children with coexisting conditions. Further studies investigating predictors of side effects in both individuals with isolated ADHD, as well as ADHD with coexisting conditions will be important.
Expanding stimulant formulations: There are now over 25 methylphenidate or amphetamine derivative stimulant medications approved by the U.S. Food and Drug Administration (see http://www.adhdmedicationguide.com/), that can vary by mode of delivery (e.g., liquid vs tab), timing of release (immediate vs extended), or stimulant derivative (e.g., methylphenidate, dexmethylphenidate, mixed amphetamine salts, lisdexamfetamine, d- & l-amphetamine). The majority of studies examined in this review used immediate release methylphenidate.
4.3. Limitations and Future Directions
Our review includes several limitations which warrant attention. First, we did not include ERP components that occurred after the P300 because few studies to date have examined these late processing components in relation to stimulant response among children with ADHD. Second, methodological heterogeneity represents a considerable limitation of research as it hinders meaningful comparisons between studies. For example, studies used a wide range of study tasks, component names, sample sizes, and time windows for analyzing ERP components (see Table 1). Further, diagnostic and inclusion criteria for research participants varied considerably across studies (see Table 1), as did the diagnostic process (e.g., parent-report form; independent rater assessment). Such methodological heterogeneity also hinders meaningful comparison between studies. Relatedly, dosing and formulation varied between the studies reviewed, and few studies systematically varied the treatments to estimate the effects of these variables on ERP components. These between-study differences (e.g., diagnostic criteria, study tasks, stimulant dosage) could, at least partially, account for some of the contradictory findings noted above. Third, the studies considered in this review often included samples that were not representative of the overall population; a majority of participants were male and Caucasian (see Table 1 for participant characteristics).
The extent to which duration of stimulant use modifies ERP components, both at baseline and following an acute dose of stimulant medication, has largely been understudied. Neuroimaging research suggests long-term stimulant use normalizes functional and structural differences in fronto-striatal circuitry among individuals with ADHD (Frodl and Skokauskas, 2012; Schweren et al., 2016). Yet, few ERP studies reviewed herein controlled for this factor. Not only will it be important to account for duration of stimulant use as a potential confound in future research, but the extent to which stimulants influence an individual’s long-term brain development constitutes another critical outcome to be measured in precision medicine care for ADHD.
Another notable concern pertains to the heterogeneity of ADHD and the degree to which studies considered such variability. Of the 42 articles reviewed herein, only seven discuss heterogeneity of ADHD as informing their methodological approach (e.g., considering demographic variables as moderators). Given that a vast majority of children (i.e., ~67%) present with comorbid disorders (e.g., anxiety, autism spectrum disorder, oppositional defiant disorder; Elia et al., 2008), such heterogeneity and related implications for medication management (Barbaresi et al., 2020). Similarly, even the most robust comparative treatment analyses have had limited power to evaluate demographic covariates of treatment response beyond characterizing patients into dichotomized pediatric versus adult age groups (Cortese et al., 2018). Accordingly, we currently know very little about how individual demographic and environmental differences contribute to stimulant medication response, let alone how these factors moderate associations between stimulant response and ERP parameters. Further, as discussed above, few studies investigate ERP responses in individuals with coexisting conditions, despite comorbidities being common in children with ADHD.
However, a few studies in the current review did acknowledge the likely neurobiological heterogeneity of their samples. Ogrim and Kropotov (2019) utilized a multimethod approach (i.e., behavioral, EEG spectra, and ERPs) to predict individual differences in behavioral response and side effects to stimulant medications with a high degree of accuracy (92% and 78%, respectively). Finally, Keage and colleagues (Keage et al., 2008) underscored the limited utility of behavior-based, diagnostic subtypes of ADHD for predicting treatment response or etiology. In this study, youth with ADHD, predominately inattentive presentation did not differ significantly from those with the combined inattentive/hyperactive-impulsive presentation on ERP amplitudes or latencies, nor were there consistent ERP differences between behavioral subtypes associated with stimulant administration.
To properly address clinical and neurophysiological heterogeneity in ADHD, very large sample sizes will be required. Thus, it will be important for clinical scientists to collaborate across sites and/or share data via publicly available datasets. This approach has been successfully used with magnetic resonance imaging (MRI) among ADHD samples and has allowed for more complex statistical analyses and modeling (see Zhang-James et al., 2019 for a discussion of ENIGMA-ADHD). However, unlike EEG/ERP, MRI methods are costly, require large, immobile equipment and are thus not feasible for use in primary care settings. Moreover, as noted by Lenartowicz and Loo (2014), sensitivity and specificity of ADHD biomarkers achieved by small EEG studies so far exceeds those reported by large fMRI datasets. We join previous researchers in the call for development of an EEG/ERP “big data” repository with diverse, representative ADHD samples to enhance the utility of ERP for development of precision medicine care in ADHD (Kiiski et al., 2020; Lenartowicz and Loo, 2014). In a similar vein, replication efforts by independent research groups are needed in order to validate candidate biomarkers.
In the effort to identify the best stimulant medication for each patient, future research should include a greater variety of stimulant medications and investigate EEG/ERP measures predictive of both improvement of ADHD symptoms and side effect profiles. Implicit in the foregoing point is the notion of prospective studies; cohort studies can assist in identifying potential biomarkers for stimulant medication response in children diagnosed with ADHD. Finally, future research could also consider characterizing multimethod biomarker profiles to optimize predictive power. A recent meta-analysis of pharmacogenetic methods predicting methylphenidate response identified genetic variants associated with better medication response (Myer et al., 2018). Accordingly, we strongly encourage taking a multimethod approach (e.g., EEG and pharmacogenetics) in development of precision medicine.
4.4. Conclusion
This review aimed to summarize what is known about ERP correlates of stimulant response in children diagnosed with ADHD. Although studies differed with regard to methods and participant characteristics, some common themes emerged. Most notably, it appears that later attention processing ERP components, such as the P300, are altered following administration of stimulant medications in youth with ADHD. More specifically, stimulant medications appear to “normalize” atypical neural activity during the action execution phase of stimulus processing by increasing recruitment and activation of cortical attention networks. Refined identification of ERP biomarkers for precision medicine care in ADHD may be achieved through use of large, representative datasets.
Highlights.
There are individual differences in stimulant response among children with attention-deficit/hyperactivity disorder (ADHD).
Scalp electrophysiology may be used to predict ADHD treatment response.
Stimulants appear to normalize the action preparation phase of attention processing.
Funding sources
This work was supported in part by grants from the National Institute of Mental Health (K99MH116064 to A.B.A.) and the Klingenstein Third Generation Foundation (ADHD Fellowship to A.B.A.).
Abbreviations:
- ADHD
Attention-Deficit/Hyperactivity Disorder
- EEG
Electroencephalography
- ERP
Event-related potential
- MPH
Methylphenidate
- TD
Typically-developing participants
- RE
medication responders
- non-RE
medication non-responders
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of Interest
None.
For example, we exclude the article by Klorman (1979) because it only reports on the late positive potential (LPP).
Several studies administered more than one cognitive task to study participants.
References
- Aasen IE, Øgrim G, Kropotov J, & Brunner JF (2018). Methylphenidate selectively modulates one sub-component of the no-go P3 in pediatric ADHD medication responders. Biol Psychol, 134, 30–38. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th Ed.). Washington, DC. [Google Scholar]
- Arns M (2012). EEG-based personalized medicine in ADHD: Individual alpha peak frequency as an endophenotype associated with nonresponse. J Neurother, 16(2), 123–141. [Google Scholar]
- Arns M, & Olbrich S (2014). Personalized medicine in ADHD and depression: use of pharmaco-EEG. In: Electrophysiology and Psychophysiology in Psychiatry and Psychopharmacology. Springer; pp. 345–370. [DOI] [PubMed] [Google Scholar]
- Banaschewski T, Brandeis D, Heinrich H, Albrecht B, Brunner E, & Rothenberger A (2003). Association of ADHD and conduct disorder–brain electrical evidence for the existence of a distinct subtype. J. Child Psychol Psychiatry, 44(3), 356–376. [DOI] [PubMed] [Google Scholar]
- Barbaresi WJ, Campbell L, Diekroger EA, Froehlich TE, Liu YH, OʼMalley E, et al. (2020). Society for Developmental and Behavioral Pediatrics Clinical Practice Guideline for the Assessment and Treatment of Children and Adolescents with Complex Attention-Deficit/Hyperactivity Disorder. J Dev Behav Pediatr, 41 Suppl 2S, 35–57. [DOI] [PubMed] [Google Scholar]
- Barry RJ, Clarke AR, & Johnstone SJ (2003). A review of electrophysiology in attention-deficit/hyperactivity disorder: I. Qualitative and quantitative electroencephalography. Clin Neurophysiol, 114(2), 171–183. [DOI] [PubMed] [Google Scholar]
- Barry RJ, Johnstone SJ, & Clarke AR (2003). A review of electrophysiology in attention-deficit/hyperactivity disorder: II. Event-related potentials. Clin Neurophysiol, 114(2), 184–198. [DOI] [PubMed] [Google Scholar]
- Blackwood D, & Muir W (1990). Cognitive brain potentials and their application. Br J Psychiatry Suppl, (9), 96–101. [PubMed] [Google Scholar]
- Brown RT, Freeman WS, Perrin JM, Stein MT, Amler RW, Feldman HM, et al. (2001). Prevalence and assessment of attention-deficit/hyperactivity disorder in primary care settings. Pediatrics, 107(3), 43–54. [DOI] [PubMed] [Google Scholar]
- Broyd SJ, Johnstone SJ, Barry RJ, Clarke AR, McCarthy R, Selikowitz M, & Lawrence CA (2005). The effect of methylphenidate on response inhibition and the event-related potential of children with attention deficit/hyperactivity disorder. Int J Psychophysiol, 58(1), 47–58. [DOI] [PubMed] [Google Scholar]
- Burcu M, Zito JM, Metcalfe L, Underwood H, & Safer DJ (2016). Trends in stimulant medication use in commercially insured youths and adults, 2010–2014. JAMA Psychiatry, 73(9), 992–993. [DOI] [PubMed] [Google Scholar]
- Clarke AR, Barry RJ, McCarthy R, & Selikowitz M (2001). EEG-defined subtypes of children with attention-deficit/hyperactivity disorder. Clin Neurophysiol, 112(11), 2098–2105. [DOI] [PubMed] [Google Scholar]
- Coons HW, Klorman R, & Borgstedt AD (1987). Effects of methylphenidate on adolescents with a childhood history of attention deficit disorder: II. Information processing. J Am Acad Child Psy, 26(3), 368–374. [DOI] [PubMed] [Google Scholar]
- Cortese S, Adamo N, Del Giovane C, Mohr-Jensen C, Hayes AJ, Carucci S, et al. (2018). Comparative efficacy and tolerability of medications for attention-deficit hyperactivity disorder in children, adolescents, and adults: a systematic review and network meta-analysis. Lancet Psychiatry, 5(9), 727–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolu N, Altınkaynak M, Güven A, Özmen S, Demirci E, İzzetoğlu M, & Pektaş F (2019). Effects of methylphenidate treatment in children with ADHD: a multimodal EEG/fNIRS approach. Psychiatr Clin Psychopharmacol, 29(3), 285–292. [Google Scholar]
- Donders FC (1969). On the speed of mental processes. Acta Psychol, 30, 412–431. [DOI] [PubMed] [Google Scholar]
- Duncan-Johnson CC (1981). Young Psychophysiologist Award Address, 1980: P300 Latency: A New Metric of Information Processing. Psychophysiology, 18(3), 207–215. [DOI] [PubMed] [Google Scholar]
- Duncan-Johnson CC, & Donchin E (1977). On quantifying surprise: The variation of event related potentials with subjective probability. Psychophysiology, 14(5), 456–467. [DOI] [PubMed] [Google Scholar]
- Elia J, Ambrosini P, & Berrettini W (2008). ADHD characteristics: I. Concurrent co-morbidity patterns in children & adolescents. Child Adolesc Psychiatry Ment Health, 2(1), 15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick PA, Klorman R, Brumaghim JT, & Borgstedt AD (1992). Effects of sustained-release and standard preparations of methylphenidate on attention deficit disorder. J Am Acad Child Psy, 31(2), 226–234. [DOI] [PubMed] [Google Scholar]
- Frank Y (1993). Visual event related potentials after methylphenidate and sodium valproate in children with attention deficit hyperactivity disorder. Clin EEG Neurosci, 24(1), 19–24. [DOI] [PubMed] [Google Scholar]
- Frodl T, & Skokauskas N (2012). Meta-analysis of structural MRI studies in children and adults with attention deficit hyperactivity disorder indicates treatment effects. Acta Psychiatr Scand, 125(2), 114–126. [DOI] [PubMed] [Google Scholar]
- Graham J, & Coghill D (2008). Adverse effects of pharmacotherapies for attention-deficit hyperactivity disorder: epidemiology, prevention and management. CNS Drugs, 22(3), 213–237. [DOI] [PubMed] [Google Scholar]
- Groom MJ, Liddle EB, Scerif G, Liddle PF, Batty MJ, Liotti M, & Hollis CP (2013). Motivational incentives and methylphenidate enhance electrophysiological correlates of error monitoring in children with attention deficit/hyperactivity disorder. J Child Psychol Psychiatry, 54(8), 836–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Group MC (2004). National Institute of Mental Health Multimodal Treatment Study of ADHD follow-up: 24-month outcomes of treatment strategies for attention-deficit/hyperactivity disorder. Pediatrics, 113(4), 754–761. [DOI] [PubMed] [Google Scholar]
- Gumenyuk V, Korzyukov O, Escera C, Hämäläinen M, Huotilainen M, Häyrinen T, et al. (2005). Electrophysiological evidence of enhanced distractibility in ADHD children. Neurosci Lett, 374(3), 212–217. [DOI] [PubMed] [Google Scholar]
- Hermens DF, Williams LM, Clarke S, Kohn M, Cooper N, & Gordon E (2005). Responses to methylphenidate in adolescent AD/HD: evidence from concurrently recorded autonomic (EDA) and central (EEG and ERP) measures. Int J Psychophysiol, 58(1), 21–33. [DOI] [PubMed] [Google Scholar]
- Hillyard SA, & Anllo-Vento L (1998). Event-related brain potentials in the study of visual selective attention. PNAS, 95(3), 781–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoza B, Kaiser N, & Hurt E (2008). Evidence-based treatments for attention-deficit/hyperactivity disorder (ADHD). In: Handbook of evidence-based therapies for children and adolescents. Springer; pp. 195–219. [Google Scholar]
- Israel J, Wickens C, Chesney G, & Donchin E (1980). The event related brain potential as a selective index of display monitoring load. Hum Factors, 22, 280–294. [DOI] [PubMed] [Google Scholar]
- Janssen TWP, Bink M, Geladé K, van Mourik R, Maras A, & Oosterlaan J (2016). A randomized controlled trial investigating the effects of neurofeedback, methylphenidate, and physical activity on event-related potentials in children with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol, 26(4), 344–353. [DOI] [PubMed] [Google Scholar]
- Johnstone IM (2001). On the distribution of the largest eigenvalue in principal components analysis. Ann Stat, 295–327. [Google Scholar]
- Jonkman LM, Kemner C, Verbaten M, Van Engeland H, Camfferman G, Buitelaar J, & Koelega H (2000). Attentional capacity, a probe ERP study: Differences between children with attention-deficit hyperactivity disorder and normal control children and effects of methylphenidate. Psychophysiology, 37(3), 334–346. [PubMed] [Google Scholar]
- Jonkman LM, Kemner C, Verbaten M, Van Engeland H, Kenemans J, Camfferman G, et al. (1999). Perceptual and response interference in children with attention-deficit hyperactivity disorder, and the effects of methylphenidate. Psychophysiology, 36(4), 419–429. [PubMed] [Google Scholar]
- Jonkman LM, Kemner C, Verbaten MN, Koelega HS, Camfferman G, vd Gaag R-J, et al. (1997). Event-related potentials and performance of attention-deficit hyperactivity disorder: children and normal controls in auditory and visual selective attention tasks. Biol Psychiatry, 41(5), 595–611. [DOI] [PubMed] [Google Scholar]
- Kaiser A, Aggensteiner P-M, Baumeister S, Holz NE, Banaschewski T, & Brandeis D (2020). Earlier versus later cognitive event-related potentials (ERPs) in attention-deficit/hyperactivity disorder (ADHD): A meta-analysis. Neurosci Biobehav Rev, 112, 117–134. [DOI] [PubMed] [Google Scholar]
- Keage HA, Clark CR, Hermens DF, Williams LM, Kohn MR, Clarke S, et al. (2008). ERP indices of working memory updating in AD/HD: differential aspects of development, subtype, and medication. J Clin Neurophysiol, 25(1), 32–41. [DOI] [PubMed] [Google Scholar]
- Kiiski H, Bennett M, Rueda-Delgado LM, Farina FR, Knight R, Boyle R, et al. (2020). EEG spectral power, but not theta/beta ratio, is a neuromarker for adult ADHD. Eur J Neurosci, 51(10), 2095–2109. [DOI] [PubMed] [Google Scholar]
- Klorman R, Brumaghim JT, Fitzpatrick PA, & Borgstedt AD (1992). Methylphenidate reduces abnormalities of stimulus classification in adolescents with attention deficit disorder. J Abnorm Psychol, 101(1), 130–138. [DOI] [PubMed] [Google Scholar]
- Klorman R, Brumaghim JT, Fitzpatrick PA, & Borgstedt AD (1990). Clinical effects of a controlled trial of methylphenidate on adolescents with attention deficit disorder. J Am Acad Child Adolesc Psychiatry, 29(5), 702–709. [DOI] [PubMed] [Google Scholar]
- Klorman R, Brumaghim JT, Fitzpatrick PA, & Borgstedt AD (1994). Clinical and cognitive effects of methylphenidate on children with attention deficit disorder as a function of aggression/oppositionality and age. J Abnorm Psychol, 103(2), 206–221. [DOI] [PubMed] [Google Scholar]
- Klorman R, Brumaghim JT, Salzman LF, Strauss J, Borgstedt AD, McBride MC, & Loeb S (1988). Effects of methylphenidate on attention-deficit hyperactivity disorder with and without aggressive/noncompliant features. J Abnorm Psychol, 97(4), 413–422. [DOI] [PubMed] [Google Scholar]
- Klorman R, Salzman LF, Bauer LO, Coons HW, Borgstedt AD, & Halpern WI (1983). Effects of two doses of methylphenidate on cross-situational and borderline hyperactive children’s evoked potentials. Clin Neurophysiol, 56(2), 169–185. [DOI] [PubMed] [Google Scholar]
- Konopka LM, & Zimmerman EM (2014). Neurofeedback and Psychopharmacology: Designing Effective Treatment Based on Cognitive and EEG Effects of Medications. In: Clinical Neurotherapy. Elsevier; pp. 55–84. [Google Scholar]
- Lawrence CA, Barry RJ, Clarke AR, Johnstone SJ, McCarthy R, Selikowitz M, & Broyd SJ (2005). Methylphenidate effects in attention deficit/hyperactivity disorder: electrodermal and ERP measures during a continuous performance task. Psychopharmacology, 183(1), 81–91. [DOI] [PubMed] [Google Scholar]
- Lenartowicz A, & Loo SK (2014). Use of EEG to diagnose ADHD. Curr Psychiatry Rep, 16(11), 498–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin AR, Naples AJ, Scheffler AW, Webb SJ, Shic F, Sugar CA, et al. (2020). Day-to-Day Test-Retest Reliability of EEG Profiles in Children With Autism Spectrum Disorder and Typical Development. Front Integr Neurosci, 14(21), 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liotti M, Pliszka SR, Perez R, Kothmann D, & Woldorff MG (2005). Abnormal brain activity related to performance monitoring and error detection in children with ADHD. Cortex, 41(3), 377–388. [DOI] [PubMed] [Google Scholar]
- Loiselle DL, Stamm JS, Maitinsky S, & Whipple SC (1980). Evoked potential and behavioral signs of attentive dysfunctions in hyperactive boys. Psychophysiology, 17(2), 193–201. [DOI] [PubMed] [Google Scholar]
- Loo SK, & Makeig S (2012). Clinical utility of EEG in attention-deficit/hyperactivity disorder: a research update. Neurotherapeutics, 9(3), 569–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo SK, McGough JJ, McCracken JT, & Smalley SL (2018). Parsing heterogeneity in attention-deficit hyperactivity disorder using EEG-based subgroups. J Child Psychol Psychiatry, 59(3), 223–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez J, Lopez V, Rojas D, Carrasco X, Rothhammer P, García R, et al. (2004). Effect of psychostimulants on distinct attentional parameters in attentional deficit/hyperactivity disorder. Biol Res, 37(3), 461–468. [DOI] [PubMed] [Google Scholar]
- Lubar JF (1991). Discourse on the development of EEG diagnostics and biofeedback for attention-deficit/hyperactivity disorders. Biofeedback Self Regul, 16(3), 201–225. [DOI] [PubMed] [Google Scholar]
- Lubar JF, Swartwood M, Swartwood J, & Timmermann D (1995). Quantitative EEG and auditory event-related potentials in the evaluation of attention-deficit/hyperactivity disorder: Effects of methylphenidate and implications for neurofeedback training. J Psychoeduc Assess, 34, 143–160. [Google Scholar]
- Luck SJ (2014). An introduction to the event-related potential technique: MIT press. [Google Scholar]
- Luck SJ, & Hillyard SA (1994). Spatial filtering during visual search: evidence from human electrophysiology. J Exp Psychol Hum Percept Perform, 20(5), 1000–1014. [DOI] [PubMed] [Google Scholar]
- Ludyga S, Brand S, Gerber M, Weber P, Brotzmann M, Habibifar F, & Pühse U (2017). An event-related potential investigation of the acute effects of aerobic and coordinative exercise on inhibitory control in children with ADHD. Dev Cog Neurosci 28, 21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luck SJ, Mathalon DH, O’Donnell BF, Hämäläinen MS, Spencer KM, Javitt DC, & Uhlhaas PJ (2011). A roadmap for the development and validation of event-related potential biomarkers in schizophrenia research. Biol Psychiat, 70(1), 28–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPartland JC, Bernier RA, Jeste SS, Dawson G, Nelson CA, Chawarska K, et al. (2020). The Autism Biomarkers Consortium for Clinical Trials (ABC-CT): Scientific Context, Study Design, and Progress Toward Biomarker Qualification. Front Integr Neurosci, 14, 16–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael RL, Klorman R, Salzman LF, Borgstedt AD, & Dainer KB (1981). Normalizing effects of methylphenidate on hyperactive children’s vigilance performance and evoked potentials. Psychophysiology, 18(6), 665–677. [DOI] [PubMed] [Google Scholar]
- Mueller V, Brehmer Y, Von Oertzen T, Li S-C, & Lindenberger U (2008). Electrophysiological correlates of selective attention: a lifespan comparison. BMC Neurosci, 9(1), 18–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myer N, Boland J, & Faraone S (2018). Pharmacogenetics predictors of methylphenidate efficacy in childhood ADHD. Mol Psychiatry, 23(9), 1929–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Näätänen R, & Picton T (1987). The N1 wave of the human electric and magnetic response to sound: a review and an analysis of the component structure. Psychophysiology, 24(4), 375–425. [DOI] [PubMed] [Google Scholar]
- Niedermeyer E, & da Silva FL (2005). Electroencephalography: basic principles, clinical applications, and related fields. Lippincott Williams & Wilkins. [Google Scholar]
- Nunez PL, & Srinivasan R (2006). Electric fields of the brain: the neurophysics of EEG. Oxford University Press, USA. [Google Scholar]
- Okazaki S, Maekawa H, Ozaki H, & Futakami S (2002). Topographic changes of ERP during a CPT-AX task at pre-and post-medication of methylphenidate in children with ADHD. In: International Congress Series. Vol. 1232. Elsevier; pp. 705–710. [Google Scholar]
- Ogrim G, Aasen IE, & Brunner JF (2016). Single-dose effects on the P3no-go ERP component predict clinical response to stimulants in pediatric ADHD. Clin Neurophysiol, 127(10), 3277–3287. [DOI] [PubMed] [Google Scholar]
- Ogrim G, & Hestad KA (2013). Effects of neurofeedback versus stimulant medication in attention-deficit/hyperactivity disorder: a randomized pilot study. J Child Adolesc Psychopharmacol, 23(7), 448–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogrim G, Kropotov J, Brunner JF, Candrian G, Sandvik L, & Hestad KA (2014). Predicting the clinical outcome of stimulant medication in pediatric attention-deficit/hyperactivity disorder: data from quantitative electroencephalography, event-related potentials, and a go/no-go test. Neuropsychiatr Dis Treat, 10, 231–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogrim G, & Kropotov JD (2019). Predicting clinical gains and side effects of stimulant medication in pediatric attention-deficit/hyperactivity disorder by combining measures from qEEG and ERPs in a Cued GO/NOGO task. Clin EEG Neurosci, 50(1), 34–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozdag M, Yorbik O, Ulas U, Hamamcioglu K, & Vural O (2004). Effect of methylphenidate on auditory event related potential in boys with attention deficit hyperactivity disorder. Int J Pediatr Otorhinolaryngol, 68(10), 1267–1272. [DOI] [PubMed] [Google Scholar]
- Papp S, Tombor L, Kakuszi B, Balogh L, Réthelyi JM, Bitter I, & Czobor P (2020). Impaired early information processing in adult ADHD: a high-density ERP study. BMC Psychiatry, 20(1), 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul-Jordanov I, Bechtold M, & Gawrilow C (2010). Methylphenidate and if-then plans are comparable in modulating the P300 and increasing response inhibition in children with ADHD. Atten Defic Hyperact Disord, 2(3), 115–126. [DOI] [PubMed] [Google Scholar]
- Petersen SE, & Posner MI (2012). The Attention System of the Human Brain: 20 Years After. Annu Rev Neurosci, 35(1), 73–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pliszka SR, Liotti M, Bailey BY, Perez III R, Glahn D, & Semrud-Clikeman M (2007). Electrophysiological effects of stimulant treatment on inhibitory control in children with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol, 17(3), 356–366. [DOI] [PubMed] [Google Scholar]
- Polich J (1986). Attention, probability, and task demands as determinants of P300 latency from auditory stimuli. Electroencephalogr Clin Neurophysiol, 63(3), 251–259. [DOI] [PubMed] [Google Scholar]
- Polich J (2012). Neuropsychology of P300. In: Luck SJ and Kappenman ES (editors). Oxford library of psychology. The Oxford handbook of event-related potential components Oxford University Press; pp. 159–188. [Google Scholar]
- Polich J, & Kok A (1995). Cognitive and biological determinants of P300: an integrative review. Biol Psychol, 41(2), 103–146. [DOI] [PubMed] [Google Scholar]
- Posner MI, & Petersen SE (1990). The Attention System of the Human Brain. Annu Rev Neurosci, 13(1), 25–42. [DOI] [PubMed] [Google Scholar]
- Prichep LS, Sutton S, & Hakerem G (1976). Evoked potentials in hyperkinetic and normal children under certainty and uncertainty: A placebo and methylphenidate study. Psychophysiology, 13(5), 419–428. [DOI] [PubMed] [Google Scholar]
- Riccio CA, Reynolds CR, Lowe P, & Moore JJ (2002). The continuous performance test: a window on the neural substrates for attention? Arch Clin Neuropsychol, 17(3), 235–272. [PubMed] [Google Scholar]
- Rubinson M, Horowitz I, Naim-Feil J, Gothelf D, Levit-Binnun N, & Moses E (2019). Effects of methylphenidate on the ERP amplitude in youth with ADHD: A double-blind placebo-controlled cross-over EEG study. PloS One, 14(5), e0217383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangal JM, & Sangal RB (2004). Attention-deficit/hyperactivity disorder: cognitive evoked potential (P300) topography predicts treatment response to methylphenidate. Clin Neurophysiol, 115(1), 188–193. [DOI] [PubMed] [Google Scholar]
- Sangal JM, Sangal RB, & Persky B (1995). Abnormal auditory P300 topography in attention deficit disorder predicts poor response to pemoline. Clin EEG Neurosci, 26(4), 204–213. [DOI] [PubMed] [Google Scholar]
- Sangal RB, & Sangal JM (2006). Attention-deficit/hyperactivity disorder: use of cognitive evoked potential (P300) to predict treatment response. Clin Neurophysiol, 117(9), 1996–2006. [DOI] [PubMed] [Google Scholar]
- Satterfield JH, Cantwell DP, Lesser LI, & Podosin RL (1972). Physiological studies of the hyperkinetic child: Am J Psychiatry, 128(11), 1418–1424. [DOI] [PubMed] [Google Scholar]
- Satterfield JH, Schell AM, Nicholas TW, Satterfield BT, & Freese TE (1990). Ontogeny of selective attention effects on event-related potentials in attention-deficit hyperactivity disorder and normal boys. Biol Psychiatry, 28(10), 879–903. [DOI] [PubMed] [Google Scholar]
- Schweren LJS, Hartman CA, Zwiers MP, Heslenfeld DJ, Franke B, Oosterlaan J et al. , (2016). Stimulant treatment history predicts frontal-striatal structural connectivity in adolescents with attention-deficit/hyperactivity disorder. Eur Neuropsychopharmacol, 26(4), 674–683. [DOI] [PubMed] [Google Scholar]
- Seifert J, Scheuerpflug P, Zillessen K-E, Fallgatter A, & Warnke A (2003). Electrophysiological investigation of the effectiveness of methylphenidate in children with and without ADHD. J Neural Transm, 110(7), 821–829. [DOI] [PubMed] [Google Scholar]
- Sibley MH, Evans SW, & Serpell ZN (2010). Social cognition and interpersonal impairment in young adolescents with ADHD. J Psychopathol Behav Assess, 32(2), 193–202. [Google Scholar]
- Smithee JAF, Klorman R, Brumaghim JT, & Borgstedt AD (1998). Methylphenidate does not modify the impact of response frequency or stimulus sequence on performance and event-related potentials of children with attention deficit hyperactivity disorder. J Abnorm Child Psychol, 26(4), 233–245. [DOI] [PubMed] [Google Scholar]
- Sobanski E, Brüggemann D, Alm B, Kern S, Deschner M, Schubert T, et al. (2007). Psychiatric comorbidity and functional impairment in a clinically referred sample of adults with attention-deficit/hyperactivity disorder (ADHD). Eur Arch Psychiatry Clin Neurosci, 257(7), 371–377. [DOI] [PubMed] [Google Scholar]
- Squires NK, Squires KC, & Hillyard SA (1975). Two varieties of long-latency positive waves evoked by unpredictable auditory stimuli in man. Electroencephalogr Clin Neurophysiol, 38(4), 387–401. [DOI] [PubMed] [Google Scholar]
- Stein MA, Sarampote CS, Waldman ID, Robb AS, Conlon C, Pearl PL, et al. (2003). A dose-response study of OROS methylphenidate in children with attention-deficit/hyperactivity disorder. Pediatrics, 112(5), 404–413. [DOI] [PubMed] [Google Scholar]
- Stroop JR (1935). Studies of interference in serial verbal reactions. J Exp Psychol, 18(6), 643–662. [Google Scholar]
- Sunohara GA, Malone MA, Rovet J, Humphries T, Roberts W, & Taylor MJ (1999). Effect of methylphenidate on attention in children with attention deficit hyperactivity disorder (ADHD): ERP evidence. Neuropsychopharmacology, 21(2), 218–228. [DOI] [PubMed] [Google Scholar]
- Sur S, & Sinha V (2009). Event-related potential: An overview. Ind Psychiatry J, 18(1), 70–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson JM, McBurnett K, Wigal T, Pfiffner LJ, Lerner MA, Williams L, et al. (1993). Effect of stimulant medication on children with attention deficit disorder: a “review of reviews”. Exceptional Children, 60(2), 154–162. [Google Scholar]
- Taylor M, Voros J, Logan W, & Malone M (1993). Changes in event-related potentials with stimulant medication in children with attention deficit hyperactivity disorder. Biol Psychol, 36(3), 139–156. [DOI] [PubMed] [Google Scholar]
- Taylor MJ, & Baldeweg T (2002). Application of EEG, ERP and intracranial recordings to the investigation of cognitive functions in children. Dev Sci, 5(3), 318–334. [Google Scholar]
- Thomas R, Sanders S, Doust J, Beller E, & Glasziou P (2015). Prevalence of attention-deficit/hyperactivity disorder: a systematic review and meta-analysis. Pediatrics, 135(4), e994–e1001. [DOI] [PubMed] [Google Scholar]
- Verbaten M, Overtoom C, Koelega H, Swaab-Barneveld H, Van der Gaag R, Buitelaar J, & Van Engeland H (1994). Methylphenidate influences on both early and late ERP waves of ADHD children in a continuous performance test. J Abnorm Child Psychol, 22(5), 561–578. [DOI] [PubMed] [Google Scholar]
- Wickens C, Kramer A, Vanasse L, & Donchin E (1983). Performance of concurrent tasks: a psychophysiological analysis of the reciprocity of information-processing resources. Science, 221(4615), 1080–1082. [DOI] [PubMed] [Google Scholar]
- Williams LM, Hermens DF, Palmer D, Kohn M, Clarke S, Keage H, et al. (2008). Misinterpreting emotional expressions in attention-deficit/hyperactivity disorder: evidence for a neural marker and stimulant effects. Biol Psychiatry, 63(10), 917–926. [DOI] [PubMed] [Google Scholar]
- Winsberg BG, Javitt DC, & Shanahan G (1997). Electrophysiological indices of information processing in methylphenidate responders. Biol Psychiatry, 42(6), 434–445. [DOI] [PubMed] [Google Scholar]
- Woodman GF (2010). A brief introduction to the use of event-related potentials in studies of perception and attention. Atten Percept Psychophys, 72(8), 2031–2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang-James Y, Helminen EC, Liu J, Franke B, Hoogman M, Faraone SV, & group E.-A. w. (2019). Machine learning classification of attention-deficit/hyperactivity disorder using structural MRI data. bioRxiv, 546671. [Google Scholar]
- Zhao X, Page TF, Altszuler AR, Pelham WE, Kipp H, Gnagy EM, et al. (2019). Family burden of raising a child with ADHD. J Abnorm Child Psychol, 47(8), 1327–1338. [DOI] [PubMed] [Google Scholar]
- Zillessen K, Scheuerpflug P, Fallgatter A, Strik W, & Warnke A (2001). Changes of the brain electrical fields during the continuous performance test in attention-deficit hyperactivity disorder-boys depending on methylphenidate medication. Clin Neurophysiol, 112(7), 1166–1173. [DOI] [PubMed] [Google Scholar]


