Abstract
Gut microbiome (GMB) has been increasingly recognized as a contributor to development and progression of heart failure (HF), immune-mediated subtypes of cardiomyopathy (myocarditis and anthracycline-induced cardiotoxicity), response to certain cardiovascular drugs, and HF-related comorbidities, such as chronic kidney disease, cardiorenal syndrome, insulin resistance, malnutrition, and cardiac cachexia. Gut microbiome is also responsible for the “gut hypothesis” of HF, which explains the adverse effects of gut barrier dysfunction and translocation of GMB on the progression of HF. Furthermore, accumulating evidence has suggested that gut microbial metabolites, including short chain fatty acids, trimethylamine N-oxide (TMAO), amino acid metabolites, and bile acids, are mechanistically linked to pathogenesis of HF, and could, therefore, serve as potential therapeutic targets for HF. Even though there are a variety of proposed therapeutic approaches, such as dietary modifications, prebiotics, probiotics, TMAO synthesis inhibitors, and fecal microbial transplant, targeting GMB in HF is still in its infancy and, indeed, requires further preclinical and clinical evidence. In this review, we aim to highlight the role gut microbiome plays in HF pathophysiology and its potential as a novel therapeutic target in HF.
Keywords: gut microbiome; heart failure; SCFA; TMAO; diet; 3,3-dimethyl-1-butanol
Introduction
Every year nearly 1 million adults in the United States are diagnosed with heart failure (HF), which is generally a result from cardiac injuries causing impairment of ventricular structure and function [1]. While we have made strides in treatment, this disease continues to have significant morbidity and mortality on par with many malignancies.
Novel preventative and therapeutic HF targets are greatly needed, and gut microbiota (GMB), a community of symbiotic bacteria, fungi and viruses residing in our gastrointestinal (GI) tract, may be a source of such targets. Growing data suggests that GMB plays an important role in a myriad of important biological processes, many of which are implicated in pathogenesis and pathophysiology of cardiomyopathy (CM) and HF and its comorbidities. In this state-of-the-art review we summarize current knowledge on HF-associated GMB dysregulation and evidence supporting the role of the GMB in pathogenesis and progression of HF and its comorbidities. We close by reviewing prospects of targeting the GMB to prevent and treat HF.
Interplay Between the Gut and the Heart - the “Gut Hypothesis” of Heart Failure
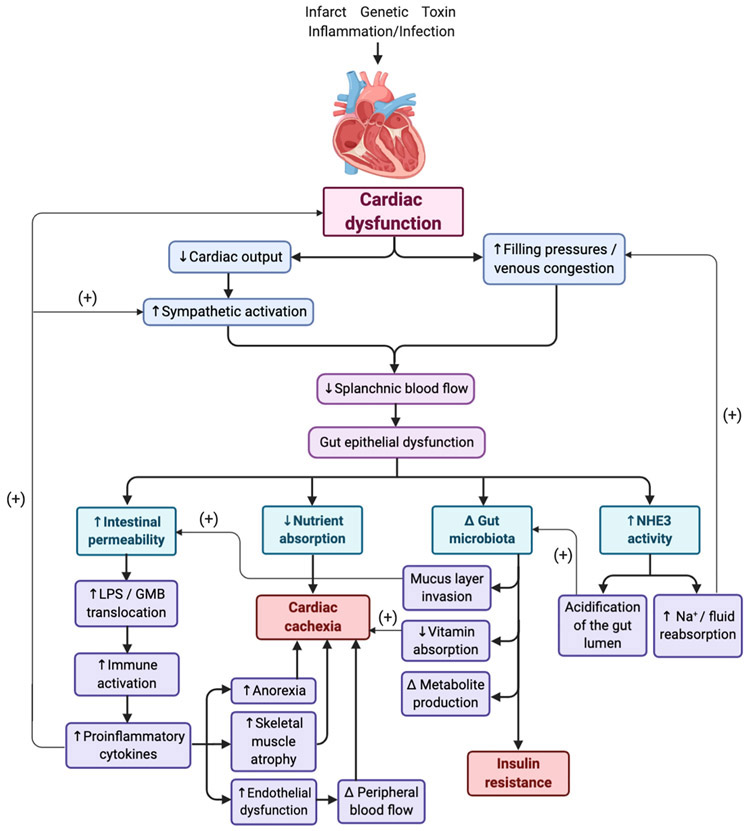
Cardiac dysfunction induces acutely adaptive, but chronically maladaptive hemodynamic, neurohumoral and pro-inflammatory responses, which both affect the gut and are propagated by it [Figure 1]. Gut ischemia develops in HF both due to elevated gut venous pressures and decreased blood flow in the splanchnic arteries [2]. The sequelae of chronic gut hypoperfusion and venous congestion include progressive interstitial edema and fibrosis of the gut wall, which correlate with HF severity, being most pronounced in patients with cardiac cachexia [3,4]. Over time these structural changes lead to functional changes, including decreased nutrient absorption and increased gut mucosal permeability [3-5]. The latter allows gut microbes and their products (such as endotoxin or lipopolysaccharide (LPS)) to more easily enter the circulation, leading to chronic low-grade inflammation characteristic of HF. The hypothesis that “leaky” gut is the main driver of systemic inflammation seen in HF is known as the “gut hypothesis” of HF. Indeed, patients with chronic decompensated HF have elevated blood levels of LPS and anti-LPS IgA antibodies, which decrease with aggressive diuretic therapy [6], and LPS levels are much higher in the portal venous system than in the left ventricle (LV) [7], supporting the hypothesis of the gut being the primary source of systemic endotoxin. Endotoxin not only triggers systemic production of pro-inflammatory cytokines, which negatively impact cardiomyocyte processes (e.g. calcium handling, mitochondrial function, etc.), cause endothelial dysfunction and impair peripheral blood flow, but it also directly impairs cardiac function by inducing intracardiac inflammatory response which damages the cardiomyocytes and decreases cardiac contractility [8]. While this mechanism is clearly pronounced in decompensated HF, intestinal permeability is also increased in patients with mild, compensated HF [5] and it is likely that there is a degree of “leaky” gut contributing to systemic inflammation at this earlier stage of disease. However, the effects of GMB in earlier stages of HF could also be mediated by mechanisms other than “leaky” gut, such as alterations in the composition of GMB community and the production of certain metabolites.
Figure 1. “Gut hypothesis” of heart failure and its comorbidities.
Figure 1 shows that hemodynamic features of heart failure (HF), decreased cardiac output and elevated intracardiac filling pressure, cause gut epithelial hypoperfusion and dysfunction, which causes increased intestinal permeability, intestinal malabsorption, altered gut microbiota, and increased sodium and fluid reabsorption, thereby resulting in increased inflammation, cardiac cachexia, insulin resistance, and HF decompensation, respectively.
Hypoperfusion-caused gut dysfunction also manifests through abnormal gut sodium and fluid handling, which is most pronounced in the setting of venous congestion and right-sided HF. Sodium-hydrogen exchanger 3 (NHE3) is the most closely regulated electrolyte channel in the gut, helping maintain balance between gut fluid and sodium secretion and absorption [9]. This channel is upregulated by epithelial intracellular hypoxia and acidosis, aldosterone and adrenal stress hormones [10-12], all of which are elevated in HF. These factors also contribute to altering the gut microbiota milieu, and it is likely that some of the subsequent alterations in the GMB community further contribute to abnormal fluid and electrolyte handling and their systemic sequelae.
Heart Failure-Associated Gut Dysbiosis - Insights from Association Studies
Marked alterations in the structure and physiology of the GI tract in HF affect resident GMB communities. Methods used in the study of the GMB are reviewed extensively elsewhere [13-15], but in Table 1 we offer a brief primer on different approaches to study of GMB. A number of studies have examined GMB differences in HF compared to healthy individuals (Table 2 provides a summary of individual study findings) [2,3,5,16-22]. While heterogeneity in findings in these studies is readily apparent, there are also shared GMB patterns more commonly seen in patients with HF compared to healthy individuals. For example, HF is associated with increased gut microbial density and invasion of the epithelial mucus layer [2,3]. The latter plays an important defensive role, preventing extensive interactions between the resident GMB and the host immune system. Relative abundance of certain strict anaerobes within the mucus biofilm in patients with HF is inversely correlated with blood flow in the inferior mesenteric artery providing blood supply to the corresponding colon segment [2]. Additionally, GMB in patients with chronic HF is characterized by overall diminished community diversity (a gross measure of community’s resilience to negative extrinsic perturbations) and depletion of microbes that have anti-inflammatory properties (mostly those belonging to the phylum Firmicutes) [16,17,19-21]. Anti-inflammatory properties of many of these bacterial taxa relate to their capacity to produce short chain fatty acids, which are byproducts of fermentation of fiber and other complex carbohydrates, and which modulate energy homeostasis, glucose metabolism, and local and systemic inflammatory responses. While the anti-inflammatory bacteria are diminished, there is an expansion of potentially pathogenic microbes (e.g. Shigella, Salmonella, Candida, etc.) in chronic HF [5,16,20]. These microbes colonize chronic HF patients more frequently than healthy individuals and at a higher abundance, and the extent of colonization correlates with HF severity [5]. Pathogen overgrowth puts HF patients at increased risk of invasive gastrointestinal infections. Indeed, a U.S. nationwide study of hospitalized, antibiotic-treated patients showed that HF was associated with significantly higher rates of concurrent Clostridioides difficile (C.difficile) infection and significantly worse in-hospital outcomes, compared to no HF [23].
Table 1.
Overview of different methods for gut microbiome characterization.
| Laboratory technique |
Description | Advantages | Disadvantages |
|---|---|---|---|
| Culturomics | - Traditional method to grow organisms in specific media and conditions | - The most sensitive method to detect the presence of known organisms - Allows detailed study on each strain - Widely available - Low cost |
- Unable to study unknown organisms - Many known organisms simply cannot be cultured - Anaerobes require advanced culture methods - Results may vary by different techniques, media, and conditions |
| Polymerase chain reaction (PCR) | - Allows identification and quantification of targeted microbial taxa and gene expression - Quantitative and reverse-transcription PCR |
- Provides absolute abundance of each taxon - Can be combined with other methods - Widely available - Relatively low cost |
- Limited to known microbes and gene - Different primers, probes, and panels can lead to different results |
| 16S rRNA gene sequencing | - Targets and amplifies hypervariable regions on 16S rRNA using specific primers followed by next-generation sequencing - Maps the sequences to a database - The most commonly used method for studying microbiome over the past decade |
- Can identify cultured and uncultured microbes at genus levels - Relatively widely accessible |
- Provides relative abundance of each genus - Lack of the detail at the species or strain level - Different primers yield significantly different microbial profiles |
| Whole metagenomics shotgun sequencing | - Sequences the entire DNA contained in a sample - Processed by advanced bioinformatics software - Displacing 16S rRNA sequencing |
- Expands the microbiome database at species- and strain-level resolution - Allows identification of functional potential of the microbial community - Increasingly available |
- Unable to provide absolute abundance - Host DNA can interfere with the results - Unknown reproducibility - Relatively expensive |
| Metatransriptomics and metaproteomics | - Sequences the complementary DNA derived from mRNA (metatranscriptomics) - Identifies proteins by mass spectrometry (metaproteomics) |
- Identifies gene expression and functional profiles of the overall microbial community | - Unable to provide results specific to each microbe - mRNA does not last long in a sample as bacterial translation takes only a few minutes - Lack of correlation between gene expression and proteins - Much more technically challenging than sequencing-based methods - Expensive |
| Metabolomics | - Identifies and quantifies non-protein metabolites of the microbial community usually using mass spectrometry - Targeted and untargeted metabolomics |
- Provides comprehensive data on inter-microbe and host-microbe interactions - Relatively widely available |
- Unable to identify genus, species, or strain of the microbe producing the metabolite - May not be sensitive for some molecules |
Table 2.
Summary of published studies of gut microbiome alterations in patients with heart failure.
| Study author (year) |
Ref. | Patients, controls, study location |
Analytical method |
Key findings in HF patients |
|---|---|---|---|---|
| Sandek et al. (2007) | [3] | 22 patients with chronic heart failure with reduced ejection fraction (HFrEF), 22 controls, Germany | Fluorescent in situ hybridization (FISH) of the sigmoid mucosal tissue biopsies | - Thousand-fold higher density of bacteria within the mucosal layer - Microbes more adherent to the mucosal biofilm |
| Sandek et al. (2014) | [2] | 65 patients with chronic HFrEF, 25 controls, Germany | FISH of stool samples and sigmoid mucosal tissue biopsies | - Similar concentration and relative abundances of bacteria in the stool samples - Increased concentration of strictly anaerobic bacteria within the mucus layer, including Eubacterium rectale, Prevotella, Faecalibacterium prausnitzii |
| Pasini et al. (2016) | [5] | 60 patients with chronic stable HF, 20 controls, Italy | Culture growth | - HF patients more frequently colonized with potential pathogens (Candida, Campylobacter, Shigella, Salmonella, Yersinia enterocolitica) and with higher relative abundances - Relative abundance of pathogens positively correlated with HF severity |
| Kamo et al. (2017) | [16] | 22 patients hospitalized for acute exacerbated HF, 12 controls, Japan | 16S rRNA gene sequencing | - Relative depletion of species Eubacterium rectale and Dorea longicatena (producers of SFCA) - Compared to younger HF patients, older HF patients had relative depletion of Faecalibacterium prausnitzii and Clostridium clostridioforme, and enrichment in Lactobacillus genus and Proteobacteria phylum (parent phylum of potentially pathogenic bacteria such as Escherichia, Shigella, etc.) |
| Luedde et al. (2017) | [17] | 20 patients with chronic HFrEF (70% with acute HF), 20 controls, Germany | 16S rRNA gene sequencing | - Lower alpha diversity - Relative depletion of families Coriobacteriaceae, Erysipelotrichaceae, Ruminococcaceae, genera Blautia and Collinsella, as well as two unclassified genera belonging to the Erysipelotrichaceae and Ruminococcaceae families |
| Kummen et al. (2018) | [19] | 84 patients with stable HFrEF (40 discovery, 44 validation cohort), 260 controls, Norway | 16S rRNA gene sequencing (for taxonomic composition), Tax4Fun (for microbial gene prediction) | - Lower GMB diversity/richness - Relative depletion of genera Faecalibacterium and Bifidobacterium, family Lachnospiraceae (including genera Blautia, Coprococcus, and species Eubacterium hallii) and depletion of in genes for butyrate production - Several taxa from the Lachnospiraceae family inversely correlated with soluble CD25, a marker of T-cell activation - Decreased abundance in Eubacterium hallii associated with worse outcomes (death or heart transplant listing) |
| Cui et al. (2018) | [20] | 53 patients with HFrEF, 41 controls, China | Metagenomic sequencing, fecal and plasma metabolomics | - Faecalibacterium prausnitzii depleted, Ruminococcus gnavus enriched - On the functional level, HF associated with decrease in the SCFA biosynthetic pathway, enrichment in TMA biosynthetic pathway and genes for LPS biosynthesis - Veillonella inversely correlated with CV protective metabolites (e.g. niacin, cinnamic acid and orotic acid), and positively correlated with sphingosine-1-phosphate (this metabolite also positively correlated with Coprobacillus and Streptococcus) |
| Katsimichas et al. (2018) | [18] | 28 patients with non-ischemic CM hospitalized with acute exacerbated HFrEF, 19 controls, Japan | 16S rRNA gene sequencing (for taxonomic composition) PICRUst (for microbial gene prediction) | - Enrichment of Streptococcus and Veillonella genera - Depletion of SMB53 genus - Many differentially abundant amino acids, carbohydrate, vitamin, and xenobiotic metabolic pathways between the two groups |
| Mayerhofer et al. (2020) | [21] | 84 patients with stable HFrEF (40 discovery, 44 validation cohort), 266 controls, Norway | 16S rRNA gene sequencing | - Lower Firmicutes/Bacteroidetes (F/B) ratio in HF, and lowest F/B ratio and alpha diversity at baseline in patients who reached a clinical endpoint (heart transplant or death) - Meat intake correlated with higher TMAO levels, and bacterial richness and abundance of several genera in the Firmicutes phylum correlated with fiber intake |
| Yuzefpolskaya et al. (2020) | [22] | 240 patients with HFrEF, chronic left ventricular assist device (LVAD) or heart transplant, No controls, United States | 16S rRNA gene sequencing Biomarkers of inflammation, endotoxemia and oxidative stress | - GMB diversity correlated inversely with severity of HF - Relative depletion of Bacteriodetes in all groups - Increased abundance of some taxa with anti-inflammatory properties, including Lachnospiraceae and Ruminococcaceae families, and Methanobrevibacter genus in patients with mild HF compared to patients with more severe HF - LPS and sCD14 higher in HF patients with most severe HF, LVAD, and heart transplant, compared to less severe HF |
There are important caveats to note when critically appraising these observed patterns of gut microbial dysbiosis in HF. Firstly, the majority of studies of GMB in patient with HF included only patients with HF with reduced ejection fraction (HFrEF). The evidence on gut dysbiosis in HF with preserved ejection fraction (HFpEF) is limited - preliminary data from Hummel et al. [24] showed that patients with HFpEF also had altered gut microbial composition compared to healthy controls, though it is not clear from that study whether GMB features that distinguished individuals with HFpEF from healthy controls mirrored those identified in studies of patients with HFrEF. Future studies would need to specifically target this group in order to better understand patterns of GMB dysregulation associated with different HF phenotypes. Secondly, certain HF-associated patterns of gut dysbiosis have been observed not only in other cardiovascular (CV) diseases (such as coronary artery disease [25,26]), but also in other chronic diseases such as metabolic syndrome and type 2 diabetes [27] and inflammatory bowel disease [28]. What these diseases have in common is a chronic, low degree, systemic inflammatory state, which has been causally tied to gut microbiome [29]. This in no way takes away from the relevance of the dysregulation patterns identified in HF, but rather implies that at least some GMB-focused therapies that will be developed in the future, targeting some of these shared GMB patterns, may find use in more than one disease.
Gut Microbiome Alterations in Heart Failure - More Than Just an Association?
While these cross-sectional studies support an association between dysregulated GMB and HF pathophysiology, they do not in themselves offer a proof of the causal link between the two. Nonetheless, while the longitudinal clinical studies are still needed, in vitro, in vivo animal studies and small translational human studies have helped elucidate the active role GMB plays in the development of CM, HF, and HF-related co-morbidities.
Gut Microbial Metabolites as Active Mediators in Heart Failure Pathophysiology
Estimated 10% of the circulating small molecules (metabolites) in mammalian blood are associated with the gut microbiota [30]. These metabolites are one of the main channels through which GMB communicates and interacts with the host. Some of these metabolites bind directly to target host receptors, while others belong to meta-organismal pathways, where a metabolite is produced by the gut microbes but is chemically modified by the host to become metabolically active. Here we review microbial metabolites with established role in HF pathophysiology. However, we also acknowledge that previously defined host-microbe interactions in the pathophysiology of HF may be just the tip of the iceberg, and a variety of gut microbial metabolites are yet to be discovered. For instance, recently, Nemet et al. demonstrated that phenylacetylglutamine, a gut microbiome-derived metabolite from phenylalanine, promoted thrombosis by activating adrenergic receptors on platelets, resulting in an increase in the risk of atherosclerotic CV disease [31].
Short Chain Fatty Acids
Fermentation of fiber, which is indigestible by the host, is one of the main functions of the gut microbiota. This process yields acetate, butyrate and propionate, known as short chain fatty acids (SCFAs). These metabolites serve as ubiquitous signaling molecules. For example, some SCFAs function as histone deacetylase inhibitors, exerting broad epigenetic effects [32]. Butyrate and propionate both help regulate vascular tone and blood pressure through receptors on smooth muscle cells [33,34]. Butyrate exerts anti-inflammatory effects through colonocyte PPAR-γ activation and regulatory T-cell proliferation [35], and, through direct modulation of colonocyte metabolism, helps prevent uncontrolled expansion of the potential pathogens [35]. Similarly, propionate was also shown to mitigate pressure-induced cardiac hypertrophy and fibrosis by maintaining immune homeostasis through regulatory T-cell activation [36].
Depletion in SCFA biosynthetic pathways and known SCFA producers (e.g. microbes from the genera Faecalibaterium, Dorea, Eubacterium, and Blautia) are cardinal features of HF-associated GMB [16,17,20]. The depletion of this group can in part explain the concomitant overgrowth of gut pathogens, development of HF-associated gastrointestinal symptoms, and possibly even HF-associated insulin resistance. Additionally, given SCFA effect on vascular smooth muscle tone, decreased SCFA production capacity within the GMB may contribute to increased cardiac afterload. Many SCFAs have a cardioprotective effect - mice fed diet lacking in fiber developed hypertension (HTN), cardiac hypertrophy and fibrosis, a phenotype that was rescued by dietary supplementation with SCFAs [37]. The SCFA mechanism of action included several G protein-coupled receptors (GPCRs), including those on T-cells localized to the gut, which mediate T-cell differentiation into anti-inflammatory regulatory T-cells [37]. In a different mice model, where high-fiber diet and SCFA acetate supplementation also led to attenuation of HTN, cardiac hypertrophy and fibrosis, the mechanisms involved downregulation of cardiac and renal early growth response 1 (Egr1), an important regulator of cardiac hypertrophy, cardiorenal fibrosis, and inflammation, and downregulation of the renin-angiotensin system in the kidney and mitogen-activated protein kinase signaling in the heart [38]. Modulation of the host immune composition and post-myocardial infarction (MI) cardiac repair capacity in mice were also mediated via SCFAs [39].
Trimethylamine N-oxide
Trimethylamine N-oxide (TMAO) is a metabolite synthesized through a well-characterized meta-organismal pathway. Choline, phosphatidylcholine, and carnitine, dietary compounds highly concentrated in foods of animal origin (such as meat, liver and eggs), are metabolized by the gut microbes into trimethylamine (TMA), and converted into TMAO in the host liver by flavin monooxygenases. Trimethylamine oxide, levels of which are virtually undetectable in vegetarians and vegans compared to omnivores [40,41], exerts pleiotropic negative cardiovascular (CV) effects. While the exact TMAO host receptor has not yet been identified, in vitro and in vivo animal and human studies have shown that TMAO modulates the cholesterol, sterol and bile acid metabolism in the liver, suppresses reverse cholesterol transport, induces platelet responsiveness, and causes vascular dysfunction, resulting in a net proatherogenic effect [40,42-46]. Ventricular remodeling is also negatively affected by TMAO [47,48]. Mice treated with supplemental TMAO or dietary TMAO precursor choline prior to development of HF experienced worsening pulmonary edema, cardiac enlargement, systolic dysfunction, and myocardial fibrosis, compared to mice receiving placebo [47].
In large human studies, TMAO levels have been independently associated with atherosclerotic heart disease and have shown to predict major adverse cardiac events [42,43,49-52]. Levels of TMAO are elevated in patients with chronic HF [53-56], correlating with functional class and diastolic, but not systolic, dysfunction [53], suggesting that increased venous congestion associated with “backward failure” might play a more significant role in altering GMB in a way that promotes TMAO production. Positive correlation between TMAO and markers of inflammation and endothelial dysfunction in HF [53] supports the notion that, in addition to the traditional “gut hypothesis” of HF, there are additional mechanisms by which GMB alterations may affect cardiac function and prognosis. Indeed, TMAO levels in patients with chronic HF are independently predictive of short- and long-term mortality [54-57].
Amino Acid Metabolites
In addition to breaking down complex carbohydrates, colonic bacteria also metabolize dietary amino acids. Indoles (including indole-3-propionate (IPA)), which are produced from dietary tryptophan by some specific gut microbes (such as Clostridium sporogenes in case of IPA [58]), fortify the intestinal barrier decreasing the gut wall permeability and circulating inflammatory cytokines [59-63]. Similar to some SCFAs, indole modulates secretion of GLP-1 to improve insulin sensitivity [64]. Patients with chronic HF, compared to healthy controls, have significantly lower levels of circulating IPA [65]. Because only certain gut bacteria carry the IPA production machinery, it is possible that specific shifts in the HF-associated GMB result in decreased capacity to produce IPA, which could then directly contribute to increased intestinal permeability and development of insulin resistance.
Microbial metabolism of dietary amino acids can lead to production of deleterious metabolites as well. For example, instead of being metabolized into IPA, tryptophan can be metabolized into indoxyl sulfate (IS). Phenylalanine and tyrosine can be metabolized into p-cresyl sulfate (pCS). These two metabolites are among best studied uremic toxins. In addition to having negative renal remodeling effects [66], these metabolites contribute to adverse cardiac remodeling through their direct pro-fibrotic, pro-hypertrophic and pro-inflammatory effects [67-69]. In dilated cardiomyopathy, high IS level has been associated with worsening of diastolic dysfunction and CV events [70]. Proposed mechanism of action of IS involves renin and angiotensin receptor activation [71,72] and induction of oxidative stress in endothelial and vascular smooth muscle cells [73-75]. Adsorption and removal of IS from the GI tract was associated with slowed progression of chronic kidney disease (CKD) [76], and lower rates of HF hospitalizations in a cohort of patients with chronic HF and comorbid CKD [77].
Bile Acids
Bile acids (BAs) are another set of metabolites relevant to CV and HF physiology that are synthesized through a set of meta-organismal pathways. Primary BAs are synthesized from cholesterol and conjugated in the host liver, before being secreted into the gut lumen where they are metabolized by the gut bacteria into secondary BAs and then largely recycled through the enterohepatic cycle. In addition to their role in fat absorption, cholesterol, lipid and glucose metabolism, BAs have a direct effect on cardiac function and vascular tone. Several BA receptors, including farnesoid X receptor, GPCR TGR5, and muscarinic M2 receptor, are expressed on cardiomyocytes, through which BAs exert differential chronotropic, inotropic and lusitropic effects [78-84]. In the vascular smooth muscle, BAs can directly stimulate calcium-activated potassium channels to regulate vascular tone and blood pressure [85].
Bile acid pool counts >50 different BAs, all with unique set of signaling roles. The composition of the GMB shapes the BA pool, modulating the potential downstream signaling effects. Bile acid pool in patients with chronic HF is distinct from that in healthy individuals. For example, a study found that chronic HF was characterized by higher ratio of secondary to primary BAs overall, driven both by lower levels of several primary BAs and higher levels of secondary BAs [86]. This study also found a modest positive correlation between plasma secondary BAs and LPS levels, suggestive of potentially increased microbial activity contributing to some of these findings [86]. A different study found that HF patients treated with ursodeoxycholate, a secondary BA thought to detoxify LPS by trapping it inside micelles, improved peripheral blood flow [87].
The Role of the Gut Microbiome in Pathogenesis of Immune-Mediated Cardiomyopathy
Not only does gut microbiome play an important role in pathogenesis of HF and cardiomyopathy through changes in its composition and metabolism, it is also implicated in the initial development of certain types of immune-mediated cardiomyopathy through interactions with the host immune system. Two such examples include myocarditis and chemotherapy-induced cardiomyopathy.
Myocarditis develops as a result of a heightened humoral or cellular immune response in the heart, usually in response to infections or drugs, or within the context of systemic autoimmune disorders. A recent study suggested that, in a mice model, particular gut commensals were critical for development of myocarditis. In transgenic mice with cardiac myosin heavy chain 6 (MYH6)-specific T-cell receptor, Bacteroides thetaiotomicron (B.theta), a common gut microbe was necessary to initiate the autoimmune process targeted against the heart [88]. B.theta produces beta-galactosidase, an enzyme that metabolizes lactose, that happens to be an MYH6 homologue. In this study beta-galactosidase induced MYH6-specific CD4+ T-cell proliferation and differentiation into pro-inflammatory Th17 cells, which then circulated systemically and infiltrated the myocardium. Notably, this study also found that patients with biopsy-proven myocarditis had higher relative abundance of B.theta in their stool compared to healthy controls, and that higher level of B.theta-specific antibodies positively correlated with disease progression and severity. However, this only occurred in patients with an HLA variant known to recognize both MYH6 and beta-galactosidase, suggesting disease development resulting from a combination of GMB and host genetic predisposition [88].
The GMB has also been implicated in pathogenesis of anthracycline-induced cardiomyopathy. These highly active chemotherapeutic agents cause intestinal epithelial damage, increasing gut permeability to LPS, while at the same time upregulating Toll-like receptor 4 (TLR4), a cellular sensor of LPS, on host macrophages and cardiomyocytes, making these cells more sensitive to LPS presence. Depletion of the GMB had equivalent effect as TLR4 signaling blockade on attenuating cardiotoxicity related to doxorubicin (DOX), a prototype anthracycline. A different study demonstrated that GMB modulation by glabridin (GLA), an isoflavone from licorice root, could prevent DOX cardiotoxicity in mice [89]. Glabridin-mediated GMB community shift was associated with increased fecal levels of SCFA butyrate, colonic anti-inflammatory macrophage polarization, and a decrease in circulating LPS levels [89]. Phenylalanine-butyrate (FBA), a synthetic derivative of SCFA butyrate, also protected mice from DOX cardiotoxicity [90], attenuating DOX-induced reactive oxygen species production. FBA exhibited similar cardioprotective properties when human induced pluripotent stem cell-derived cardiomyocytes were treated with DOX. Reassuringly, this synthetic butyrate derivative did not compromise, but in fact enhanced tumor blocking effects of DOX in tested breast cancer cells [90]. These benefits of FBA may be from the effects of butyrate on attenuating cardiac apoptosis and remodeling by inhibiting histone deacetylases and reducing endoplasmic reticulum stress [91,92].
These examples demonstrate that GMB alterations play a pathogenic role in development of cardiomyopathy in specific disease and animal models, that the mechanisms at least in part involve interaction with the host immune system, that these interactions may be modified by host genotype, and that active modulation of the GMB can have cardioprotective effects.
Heart Failure Comorbidities and the Role of the Gut Microbiota
Gut microbiome contributes not only to the development of cardiomyopathy and HF and to progression of HF but is also an active mediator in the pathogenesis of several conditions that develop as complications of HF, which increase the morbidity and mortality of patients with HF. Understanding how GMB is linked to HF-associated comorbidities may provide more insight into complex host-microbe relationships, which can serve as promising therapeutic targets.
Chronic Kidney Disease and Cardiorenal Syndrome
More than half of all patients with chronic HF have co-morbid CKD [93]. Maladaptive activation of the sympathetic and renin-angiotensin-aldosterone systems, systemic inflammation, and ineffective fluid handling are aspects of pathophysiology of both conditions, and each condition accelerates disease progression in the other, setting up a vicious cycle [94]. Interestingly, the gut and the commensal GMB may play an important role in signaling between the two organs. Firstly, similarly to HF, CKD can contribute to the disruption of the intestinal mucosal barrier - ammonia and ammonium hydroxide, products of the microbial metabolism of accumulated urea, are toxic to intestinal epithelial cells [95], which promotes bacterial translocation and systemic inflammation. Secondly, TMAO has been mechanistically linked to development of both HF and CKD. As detailed previously, this gut microbial metabolite is pro-atherogenic and has negative effects on ventricular remodeling and myocardial fibrosis. It also directly contributes to progressive renal fibrosis and dysfunction in animal models [96] and has been associated with worse long-term prognosis in patients with CKD [97]. Lastly, IS and pCS, products of the GMB metabolism of dietary amino acids, have pro-fibrotic, pro-hypertrophic, and pro-inflammatory effects on the heart, in addition to their general pro-thrombotic effects and role in endothelial dysfunction [66].
Insulin Resistance
Insulin resistance (IR) is both an independent risk factor for development of cardiomyopathy [98,99] and a prevalent metabolic complication of chronic HF, affecting more than half of all patients with HF [100]. It is a comorbidity that progresses in parallel to HF severity [101,102] and independently portends worse overall long-term survival in HF [103,104]. Insulin resistance contributes to the progression of HF through direct toxic effects on the cardiac muscle and indirectly through negative systemic effects [105]. For the individuals who develop IR after onset of HF, the mechanism by which IR develops has not yet been fully elucidated [106,107]. Given that the altered GMB has been implicated in the development of IR, diabetes, and metabolic syndrome in animal and human non-HF models [27,108-110], it is conceivable that specific patterns of GMB dysregulation might contribute to development of IR within the context of HF and to the development of CM and HF in individuals with pre-existing IR. Indeed, there are significant overlaps between the GMB and the associated circulating metabolome signatures of HF and isolated IR. For example, similar to patients with chronic HF, GMB of patients with IR and metabolic syndrome is depleted of SCFA-producers such as Faecalibacterium prausnitzii and Eubacterium rectale and enriched in potentially pathogenic bacteria such as Escherichia coli [27,109,110]. Similarly, HF and IR are associated with increased levels of branched chain amino acids (BCAAs) (leucine, isoleucine, and valine) and their catabolites [111-115]. The capacity to synthesize and handle these metabolites, which were found to directly contribute to development of IR in multiple rodent studies [113,115], has been closely related to the GMB [115]. Other gut microbial metabolites have also been shown to be associated with IR, including SCFA [116,117], TMAO [118,119], and BAs [120-122]. Future research needs to further explore the connections between HF and IR mediated by alterations in GMB structure and function.
Malnutrition and Cardiac Cachexia
Chronic HF is a catabolic state, which culminates in development of cardiac cachexia. The gut and the GMB have been implicated in this process [2,123,124]. Abnormal intestinal permeability, driven primarily by altered splanchnic blood flow, is the principal mechanism by which cardiac cachexia develops. Increased intestinal epithelial permeability results in translocation of the gut bacteria and their products, initiating and maintaining the pro-inflammatory milieu of HF. Elevated cytokines such as IL-6 and TNF-α, which are highest in HF patients with comorbid cardiac cachexia, have an anorectic effect [125]. Furthermore, they also mediate skeletal myocyte apoptosis and endothelial dysfunction associated with decreased blood supply to skeletal muscles, which results in skeletal muscle wasting and sarcopenia [126].
The gut microbiome is capable of directly regulating the bowel wall permeability, thereby potentially contributing to both HF and cachexia. For example, one study showed that colonization with Escherichia coli, Klebsiella pneumoniae, and Streptococcus viridans significantly increased bowel permeability in rats, whereas colonization with Lactobacillus brevis had the opposite effect [127]. Additionally, higher degree of adherence of commensal bacteria to the gut epithelium disrupts the gut epithelial barrier function and stimulates mucosal cytokine production [128], which is pertinent in HF because gut microbes in patients with HF adhere to the epithelial layer more tightly and invade the mucus layer more readily [3] than in healthy controls. In addition to increased epithelial barrier permeability, gut dysfunction in patients with HF (especially those with cardiac cachexia) also causes significant protein and fat malabsorption. While there is a lack of data showing that gut microbes are directly involved in this process, it is known that pathogenic gut bacterial overgrowth, characteristic of chronic HF, may impair metabolic efficiency by reducing intestinal absorptions of vitamin B12, folate, and vitamin K, which are essential for protein metabolism.
Although it is plausible that HF-related GMB dysbiosis and dysfunction play an important role in developing cardiac cachexia, more preclinical and clinical studies are needed in order to verify this hypothesis and elucidate mechanisms of host-microbiota interactions in these processes before we can employ GMB-focused approaches for prevention and treatment of cardiac cachexia.
Microbial-Drug Interactions and Implications for Heart Failure Therapeutics
While inter-individual variability in drug pharmacokinetics and pharmacodynamics caused by host genetic make-up (pharmacogenomics) has been well recognized, there is growing data to support resident GMB contributions to this phenomenon (pharmacomicrobiomics). Resident GMB and its metabolites can alter drug absorption and (in)activation. They can also compete for drug-metabolizing enzymes in the liver and alter their expression [129]. The drug-microbiome interactions are bidirectional - drugs also alter the composition and activity of the GMB community [130].
Many of the drugs used for treatment of HF and CV disease interact with the GMB. One of them is digoxin, a natural cardiac glycoside used in treatment of HF and atrial fibrillation and a drug infamous for its variability in its effectiveness and toxicity. Digoxin is metabolized into an inactive metabolite exclusively by the gut commensal Eggerthella lenta (E.lenta) [131]. In fact, only specific strains of E.lenta, those carrying the cardiac glycoside reductase (cgr) operon, are able to inactivate digoxin [132]. The expression of the cgr operon in mice is downregulated by amino acid arginine and a protein-rich diet, leading to increased serum and urine digoxin levels [132]. Some of the other CV drugs that interact with the GMB include aspirin, beta blockers, angiotensin-converting enzyme and angiotensin receptor inhibitors, and calcium channel blockers (CCB) [70,71,74]. A recent study confirmed that several of these drug classes are metabolized by a number of common gut bacteria. For example, deacetylation of diltiazem was found to be specifically dependent on bt4096 gene-carrying gut commensal B.theta [129]. Amlodipine also undergoes GMB-mediated biotransformation, and it has been shown that pre-treatment with ampicillin is associated with significantly higher circulating drug levels in rats [133]. These studies suggest that inter-individual variability in CV drug response observed in clinical practice may, at least in part, be mediated by individual GMB composition and can be further modified by individual GMB-dietary interactions.
Targeting the Gut Microbiome to Prevent and Treat Heart Failure - Are We There Yet?
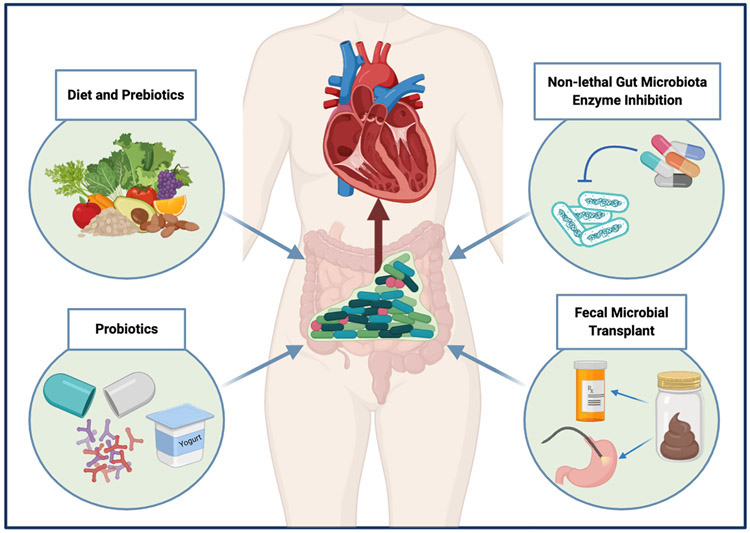
Different approaches to GMB modulation - ranging from broad and non-specific dietary interventions and fecal microbiota transplant (FMT) to ones involving specific and targeted microbial enzyme inhibition - have been proposed in many diseases. Given the role that the GMB plays in development and progression of cardiomyopathy and HF, development of its comorbidities, and modulation of the effects of HF therapy, the prospect of GMB as a preventative and therapeutic target in HF is being seriously considered. While larger and more comprehensive clinical studies will be needed before any of these interventions are adopted in clinical practice, early data demonstrating effects of GMB manipulation in animal HF models and small human studies is promising and reviewed here. Here we review some of the data supporting proposed approaches to GMB modulation in HF (also illustrated in Figure 2).
Figure 2. Proposed approaches to modulating gut microbiota in heart failure.
Figure 2 illustrates different approaches to modulation of the gut microbiota in heart failure. These include dietary, prebiotic and probiotic interventions, targeted non-lethal inhibition of enzymes within the gut microbiota, and fecal microbiota transplantation.
Dietary and Prebiotic Interventions
Diet is one of the factors that most profoundly impacts the GMB - it is likely that GMB at least partially mediates cardioprotective effects of certain dietary patterns. Mediterranean diet, a diet typically rich in unprocessed or minimally processed vegetables, fruits, nuts, legumes, fiber and complex carbohydrates, fish, and healthy fats, and low in meat (especially red meat) and meat products, refined sugars and processed foods, has been shown to reduce risk of development and progression of HF and atherosclerotic heart disease. Multicenter, randomized PREDIMED study found that the traditional Mediterranean diet was associated with approximately 30% relative reduction in MI, stroke, or CV death after a nearly 5-year follow-up [134]. Mediterranean diet has also been associated with decreased risk of incident HF [135,136].
Interestingly, it is possible that CV benefits from Mediterranean diet are from a non-lethal TMA lyase inhibitor, 3,3-dimethyl-1-butanol (DMB), which is naturally found in extra virgin olive oil widely used in Mediterranean diet [137,138]. Moreover, dietary Approaches to Stop Hypertension (DASH) diet and other plant-based diets, which are in many ways similar to the Mediterranean diet, were also associated with decreased risk of incident HF [136,139-141]. Additionally, many of these healthy eating patterns have been associated with HF severity and clinical outcomes [142-144].
These plant-based diets are rich in fiber and complex carbohydrates, which are known as prebiotics. Prebiotics are food components and supplements that cannot be digested by the host and are metabolized primarily by our GMB. Availability of fiber and other prebiotics in diet is beneficial in several ways. In its presence, gut microbes will preferentially ferment dietary fiber, instead of digesting through our protective gut mucus lining [145], which has an overall anti-inflammatory effect for the host and helps preserve insulin sensitivity [146]. Additional, fermentation of fiber yields SCFAs, which then directly affect host energy homeostasis, insulin sensitivity, blood pressure, and overall immune system balance, as described above.
The effects of diet, including Mediterranean and plant-based diets, on the GMB composition have been studied in different populations. These studies have shown generally positive effects on GMB diversity and abundance of microbes known for their fibrolytic activity, SCFA production, and downstream anti-inflammatory host effects [147]. In one study of HF patients, researchers found that fiber intake was associated with increased bacterial richness and abundance of several genera from the Firmicutes phylum [21], postulating that the gut dysregulation in HF may in part be related to low fiber intake, suggesting dietary modifications as potential therapeutic interventions in HF. Beneficial effects of fiber supplementation in HF have been demonstrated in mice studies. For example, one study found that fiber-depleted diet in mice subjected to a mild hypertensive stimulus resulted in development of HTN, cardiac hypertrophy and fibrosis. Fiber supplementation, but also SCFA supplementation alone, rescued this phenotype [38]. Both interventions modified GMB composition, increasing the abundance of SCFA acetate-producing bacteria and decreasing the level of gut dysbiosis [38]. It is important to note that the cardiovascular benefits of the Mediterranean and plant-based diets both in general population and on HF patients were identified in population-level studies that did not include concomitant analysis of GMB. Therefore, the proposition that some of the benefits of these diets are mediated by the gut microbiome is at this point only a hypothesis, which should be evaluated in dedicated interventional studies.
Diet-gut microbiome interactions may also result in generation of cardiotoxic metabolites. For example, dietary L-carnitine- and choline-derived microbial metabolite TMAO is much higher in omnivores than in individuals consuming vegetarian and vegan diets [40]. Red meat is particularly high in TMAO precursors, but, reassuringly, dramatic reduction in circulating TMAO levels can be achieved after only few weeks of cessation of red meat intake [41]. It is possible that some of the CV benefits of Mediterranean and plant-based diets may be related to low meat intake and decreased production of cardiotoxic metabolites. Meat intake is positively associated with TMAO in patients with HF as well [21].
Salt intake is another aspect of diet that affects GMB composition and its metabolic output. Wilck et al. showed that high salt diet (HSD) in mice led to depletion of the gut microbe Lactobacilllus murinus, and subsequent supplementation with this microbe increased GMB output of indole (a metabolite of dietary tryptophan), suppressed pro-inflammatory Th17 cells proliferation, and attenuated development of HTN [148]. Similar findings were noted in a small human pilot study, where a two-week course of HSD resulted in near complete loss of Lactobacillus, expansion of circulating Th17 cells and elevated blood pressure [148]. Other studies have similarly demonstrated HSD-related alterations of the GMB composition and change in SCFA output [149].
Restriction of salt intake is the cornerstone of HF management and endorsed by multiple guidelines, and it is likely that some benefits of low sodium diet in HF may be mediated via GMB, as suggested by the above studies. However, findings of other studies reviewed here suggest that individuals at risk of HF or already diagnosed with this disease may potentially benefit from more specific dietary guidelines. It is also likely that more comprehensive and granular data will in the future enable us to provide our HF patients with truly personalized nutritional recommendations that will be derived from both their unique GMB signature and their genotype.
Probiotic Interventions
Probiotics are live microbes ingested by the host that can interact with the resident GMB community, resulting in altered community balance and metabolic output. Potential benefit of probiotics in HF has been demonstrated mostly in animal studies. Supplementation with Lactobacillus rhamnosus GR-1 in rats suffering from induced ischemic HF resulted in attenuation of LV hypertrophy, improvement in systolic and diastolic LV function and hemodynamic parameters [150]. Notably, observed probiotic-related benefits persisted for weeks following treatment cessation. In a different study, pre-treatment of rats with either oral vancomycin or probiotic Lactobacillus plantarum 299v/Bifidobacterium lactis Bi-07 prior to induced MI resulted in significantly smaller infarct area and overall improved cardiac function [151]. Cardioprotective effects of Lactobacillus supplementation were also seen in mice with induced MI and HF pre-treated with broad-spectrum antibiotics [39].
The only published human study of effects of probiotics in HF to date was a small randomized study, where HF patients received either a 3-month course of probiotic Saccharomyces boulardii (S.boulardii) or placebo [152]. Probiotic treatment was associated with statistically significant, albeit modest, improvement in LV systolic function and left atrial size. More data are coming - GutHeart in an ongoing randomized, open-label, controlled trial of patients with stable HF receiving either S.boulardii, antibiotic rifaximin, or no treatment [153,154]. Primary end-point of the study will be change in LV systolic function, but the study will also evaluate changes in quality of life, functional capacity, and biomarkers of gut permeability and systemic inflammation.
Gut Microbial Enzyme Inhibition
The obvious limitation of several of the discussed therapeutic approaches is the lack of specificity, which may result in unintended and potentially negative host effects. Many of the gut microbial enzymes are not found in the host, so targeting these enzymes is a very elegant way to overcome these challenges. Additionally, doing so in a non-lethal fashion may avoid unintended negative effects on the remainder of the GMB community. So far, this approach has been used in targeting the TMAO meta-organismal pathway. 3,3-dimethy-1-lbutanol is a non-lethal inhibitor of TMA lyases, bacterial enzymes that help convert dietary choline to TMA. Its administration in mice fed a high choline diet resulted in decreased circulating TMAO levels, decreased foam cell formation and fewer atherosclerotic plaques [155]. Interestingly, DMB is naturally found in extra virgin olive oil, balsamic vinegar and red wine [155], staples of the Mediterranean diet. Inhibition of other enzymes in the TMA/TMAO synthetic pathway also resulted in complete suppression of choline diet-enhanced TMAO generation, as well as attenuated platelet aggregation and artery clot formation in mice [156]. Targeted inhibition of microbial choline-TMAO conversion was also evaluated in pressure overload mice model of HF, where it improved cardiac remodeling and cardiac function [157]. While human studies evaluating the feasibility and efficacy of this elegant approach are needed, studies reviewed here suggest that both pharmacologic modification of the TMAO biosynthetic pathway and targeted dietary interventions may be viable strategies for modulating pathogenesis and progression of HF.
Fecal Microbiota Transplant
Fecal microbiota transplant involves transfer of stool from a healthy individual into diseased recipient’s GI tract. This approach has been tremendously successful in treatment of recurrent, antibiotic-resistant C.difficile infection [158]. While FMT is presently approved only for this indication, preliminary data suggests that this approach may be beneficial for many other diseases and is currently being evaluated in hundreds of clinical trials, both in gastrointestinal and systemic diseases. For example, FMT has shown promise in treating IR and metabolic syndrome [110,159]. It is likely, however, that moving forward this approach will be met with increased scrutiny given several recent cases where several immunocompromised FMT recipients died after developing systemic infection caused by organisms originating from the donor GMB which were not identified on initial donor screening [160]. More comprehensive donor screening protocols will need to be developed to avoid serious adverse effects. As of writing of this article, there have been no studies of FMT in HF, but, provided that the above challenges are addressed, this may be a feasible option in the future.
Conclusions
Accumulating evidence has suggested that GMB plays an important role in development and progression of HF, its comorbidities, and immune-mediated subtypes of cardiomyopathy, including myocarditis and anthracycline-induced cardiotoxicity. Microbial translocation, and altered structure and function of GMB have been consistently shown to contribute to HF, emphasizing the intense interaction between the gut and heart, known as the “gut hypothesis” of HF. Over the past decade, metabolomics has become one of the most commonly used methods for studying metabolic function and output of GMB. Discovery of metabolites such as SCFA, TMAO, amino acid metabolites, and BAs has enabled elucidation of complex host-microbe interactions in HF and other diseases, and these metabolites serve as promising therapeutic targets for HF. Nevertheless, the evidence on the causal relationship between altered GMB and HF is mostly from preclinical studies, and the proposed mechanistic links still need to be confirmed in clinical studies. Moreover, there is still limited evidence on efficacy and safety of direct modifications of the gut microbial composition and metabolic function, especially in humans. Therefore, further investigations need to focus on expanding insight into the role of GMB in the pathogenesis of HF, and developing novel therapeutic interventions targeting GMB in HF.
Acknowledgments
Funding Support
Dr. Mamic is partially supported by a grant from the National Institutes of Health (F32HL143916). Dr. Tang is partially supported by grants from the National Institutes of Health (R01DK106000, R01HL126827).
Abbreviations:
- BAs
Bile Acids
- CCB
Calcium Channel Blockers
- CKD
chronic kidney disease
- CM
Cardiomyopathy
- CV
Cardiovascular
- DASH
Dietary Approaches to Stop Hypertension
- DMB
3,3-dimethyl-1-butanol
- DOX
Doxorubicin
- Egr1
Early Growth Response 1
- FBA
Phenylalanine-butyrate
- FMT
Fecal Microbial Transplant
- GI
Gastrointestinal
- GLA
Glabridin
- GMB
Gut Microbiome
- GPCRs
G Protein-Coupled Receptors
- HF
Heart Failure
- HTN
Hypertension
- IPA
Indole-3-propionate
- IR
Insulin Resistance
- IS
Indoxyl Sulfate
- LPS
Lipopolysaccharide
- LV
Left Ventricle
- MI
Myocardial Infarction
- MYH6
Myosin Heavy Chain 6
- NHE3
Sodium-Hydrogen Exchanger 3
- pCS
p-Cresyl Sulfate
- SCFAs
Short Chain Fatty Acids
- TLR4
Toll-like Receptor 4
- TMA
Trimethylamine
- TMAO
Trimethylamine N-oxide
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure
Dr. Tang is a consultant for Sequana Medical A.G., Owkin Inc, and Relypsa Inc, and has received honorarium from Springer Nature for authorship/editorship and American Board of Internal Medicine for exam writing committee participation, all unrelated to the contents of this paper. All other authors have no relationships to disclose, both unrelated to the contents of this paper.
References
- 1.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Drazner MH, et al. 2013 ACCF/AHA Guideline for the Management of Heart Failure. Journal of the American College of Cardiology. 2013. October 15;62(16):e147–239. [DOI] [PubMed] [Google Scholar]
- 2.Sandek A, Swidsinski A, Schroedl W, Watson A, Valentova M, Herrmann R, et al. Intestinal Blood Flow in Patients With Chronic Heart Failure: A Link With Bacterial Growth, Gastrointestinal Symptoms, and Cachexia. J Am Coll Cardiol. 2014. September 16;64(11):1092–102. [DOI] [PubMed] [Google Scholar]
- 3.Sandek A, Bauditz J, Swidsinski A, Buhner S, Weber-Eibel J, von Haehling S, et al. Altered Intestinal Function in Patients With Chronic Heart Failure. Journal of the American College of Cardiology. 2007. October 16;50(16):1561–9. [DOI] [PubMed] [Google Scholar]
- 4.Arutyunov GP, Kostyukevich OI, Serov RA, Rylova NV, Bylova NA. Collagen accumulation and dysfunctional mucosal barrier of the small intestine in patients with chronic heart failure. International Journal of Cardiology. 2008. April 10;125(2):240–5. [DOI] [PubMed] [Google Scholar]
- 5.Pasini E, Aquilani R, Testa C, Baiardi P, Angioletti S, Boschi F, et al. Pathogenic Gut Flora in Patients With Chronic Heart Failure. JACC: Heart Failure. 2016. March 1;4(3):220–7. [DOI] [PubMed] [Google Scholar]
- 6.Niebauer J, Volk H-D, Kemp M, Dominguez M, Schumann RR, Rauchhaus M, et al. Endotoxin and immune activation in chronic heart failure: a prospective cohort study. The Lancet. 1999. May 29;353(9167):1838–42. [DOI] [PubMed] [Google Scholar]
- 7.Peschel T, Schönauer M, Thiele H, Anker SD, Schuler G, Niebauer J. Invasive assessment of bacterial endotoxin and inflammatory cytokines in patients with acute heart failure. Eur J Heart Fail. 2003. October;5(5):609–14. [DOI] [PubMed] [Google Scholar]
- 8.Rogler G, Rosano G. The heart and the gut. Eur Heart J. 2014. February 14;35(7):426–30. [DOI] [PubMed] [Google Scholar]
- 9.Bookstein C, DePaoli AM, Xie Y, Niu P, Musch MW, Rao MC, et al. Na+/H+ exchangers, NHE-1 and NHE-3, of rat intestine. Expression and localization. J Clin Invest. 1994. January 1;93(1):106–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kiela PR, Guner YS, Xu H, Collins JF, Ghishan FK. Age- and tissue-specific induction of NHE3 by glucocorticoids in the rat small intestine. American Journal of Physiology-Cell Physiology. 2000. April 1;278(4):C629–37. [DOI] [PubMed] [Google Scholar]
- 11.Musch MW, Lucioni A, Chang EB. Aldosterone regulation of intestinal Na absorption involves SGK-mediated changes in NHE3 and Na+ pump activity. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2008. November 1;295(5):G909–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lucioni A, Womack C, Musch MW, Rocha FL, Bookstein C, Chang EB. Metabolic acidosis in rats increases intestinal NHE2 and NHE3 expression and function. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2002. July 1;283(1):G51–6. [DOI] [PubMed] [Google Scholar]
- 13.Morgan XC, Huttenhower C. Meta’omic Analytic Techniques for Studying the Intestinal Microbiome. Gastroenterology. 2014. May 1;146(6):1437–1448.e1. [DOI] [PubMed] [Google Scholar]
- 14.Allaband C, McDonald D, Vázquez-Baeza Y, Minich JJ, Tripathi A, Brenner DA, et al. Microbiome 101: Studying, Analyzing, and Interpreting Gut Microbiome Data for Clinicians. Clinical Gastroenterology and Hepatology. 2019. January 1;17(2):218–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lagier J-C, Armougom F, Million M, Hugon P, Pagnier I, Robert C, et al. Microbial culturomics: paradigm shift in the human gut microbiome study. Clinical Microbiology and Infection. 2012. December 1;18(12):1185–93. [DOI] [PubMed] [Google Scholar]
- 16.Kamo T, Akazawa H, Suda W, Saga-Kamo A, Shimizu Y, Yagi H, et al. Dysbiosis and compositional alterations with aging in the gut microbiota of patients with heart failure. PLoS ONE. 2017;12(3):e0174099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luedde M, Winkler T, Heinsen F-A, Rühlemann MC, Spehlmann ME, Bajrovic A, et al. Heart failure is associated with depletion of core intestinal microbiota. ESC Heart Failure. 2017;4(3):282–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Katsimichas T, Ohtani T, Motooka D, Tsukamoto Y, Kioka H, Nakamoto K, et al. Non-Ischemic Heart Failure With Reduced Ejection Fraction Is Associated With Altered Intestinal Microbiota. Circ J. 2018. 25;82(6):1640–50. [DOI] [PubMed] [Google Scholar]
- 19.Kummen M, Mayerhofer CCK, Vestad B, Broch K, Awoyemi A, Storm-Larsen C, et al. Gut Microbiota Signature in Heart Failure Defined From Profiling of 2 Independent Cohorts. J Am Coll Cardiol. 2018. March 13;71(10):1184–6. [DOI] [PubMed] [Google Scholar]
- 20.Cui X, Ye L, Li J, Jin L, Wang W, Li S, et al. Metagenomic and metabolomic analyses unveil dysbiosis of gut microbiota in chronic heart failure patients. Scientific Reports. 2018. January 12;8(1):635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mayerhofer CCK, Kummen M, Holm K, Broch K, Awoyemi A, Vestad B, et al. Low fibre intake is associated with gut microbiota alterations in chronic heart failure. ESC Heart Failure. 2020;7(2):456–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yuzefpolskaya M, Bohn B, Nasiri M, Zuver AM, Onat DD, Royzman EA, et al. Gut microbiota, endotoxemia, inflammation, and oxidative stress in patients with heart failure, left ventricular assist device, and transplant. The Journal of Heart and Lung Transplantation. 2020. September 1;39(9):880–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mamic P, Heidenreich PA, Hedlin H, Tennakoon L, Staudenmayer KL. Hospitalized Patients with Heart Failure and Common Bacterial Infections: A Nationwide Analysis of Concomitant Clostridium Difficile Infection Rates and In-Hospital Mortality. Journal of Cardiac Failure. 2016. November 1;22(11):891–900. [DOI] [PubMed] [Google Scholar]
- 24.Hummel Scott L, Bassis Christine, Marolt Cara, Konerman Matthew, Schmidt Thomas M GUT MICROBIOME DIFFERS BETWEEN HEART FAILURE WITH PRESERVED EJECTION FRACTION AND AGE-MATCHED CONTROLS. Journal of the American College of Cardiology. 2019. March 12;73(9_Supplement_1):750–750. [Google Scholar]
- 25.Trøseid M, Andersen GØ, Broch K, Hov JR. The gut microbiome in coronary artery disease and heart failure: Current knowledge and future directions. EBioMedicine. 2020. February;52:102649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tang WHW, Kitai T, Hazen SL. Gut Microbiota in Cardiovascular Health and Disease. Circulation Research. 2017. March 31;120(7):1183–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qin J, Li Y, Cai Z, Li S, Zhu J, Zhang F, et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012. October;490(7418):55–60. [DOI] [PubMed] [Google Scholar]
- 28.Khan I, Ullah N, Zha L, Bai Y, Khan A, Zhao T, et al. Alteration of Gut Microbiota in Inflammatory Bowel Disease (IBD): Cause or Consequence? IBD Treatment Targeting the Gut Microbiome. Pathogens. 2019. August 13;8(3):126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang J, Chen W-D, Wang Y-D. The Relationship Between Gut Microbiota and Inflammatory Diseases: The Role of Macrophages. Front Microbiol. 2020. June 9;11:1065–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wikoff WR, Anfora AT, Liu J, Schultz PG, Lesley SA, Peters EC, et al. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. PNAS. 2009. March 10;106(10):3698–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nemet I, Saha PP, Gupta N, Zhu W, Romano KA, Skye SM, et al. A Cardiovascular Disease-Linked Gut Microbial Metabolite Acts via Adrenergic Receptors. Cell. 2020. March 5;180(5):862–877.e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Davie JR. Inhibition of Histone Deacetylase Activity by Butyrate. J Nutr. 2003. July 1;133(7):2485S–2493S. [DOI] [PubMed] [Google Scholar]
- 33.Pluznick JL, Protzko RJ, Gevorgyan H, Peterlin Z, Sipos A, Han J, et al. Olfactory receptor responding to gut microbiota-derived signals plays a role in renin secretion and blood pressure regulation. PNAS. 2013. March 12;110(11):4410–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pluznick J A novel SCFA receptor, the microbiota, and blood pressure regulation. Gut Microbes. 2014. March 1;5(2):202–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Byndloss MX, Olsan EE, Rivera-Chávez F, Tiffany CR, Cevallos SA, Lokken KL, et al. Microbiota-activated PPAR-γ-signaling inhibits dysbiotic Enterobacteriaceae expansion. Science. 2017. August 11;357(6351):570–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bartolomaeus H, Balogh A, Yakoub M, Homann S, Markó L, Höges S, et al. Short-Chain Fatty Acid Propionate Protects From Hypertensive Cardiovascular Damage. Circulation. 2019. March 12;139(11):1407–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaye DM, Shihata WA, Jama HA, Tsyganov K, Ziemann M, Kiriazis H, et al. Deficiency of Prebiotic Fiber and Insufficient Signaling Through Gut Metabolite-Sensing Receptors Leads to Cardiovascular Disease. Circulation. 2020. April 28;141(17):1393–403. [DOI] [PubMed] [Google Scholar]
- 38.Marques FZ, Nelson E, Chu P-Y, Horlock D, Fiedler A, Ziemann M, et al. High-Fiber Diet and Acetate Supplementation Change the Gut Microbiota and Prevent the Development of Hypertension and Heart Failure in Hypertensive Mice. Circulation. 2017. March 7;135(10):964–77. [DOI] [PubMed] [Google Scholar]
- 39.Tang Tony WH, Chen Hung-Chih, Chen Chen-Yun, Yen Christopher YT, Lin Chen-Ju, Prajnamitra Ray P, et al. Loss of Gut Microbiota Alters Immune System Composition and Cripples Postinfarction Cardiac Repair. Circulation. 2019. January 29;139(5):647–59. [DOI] [PubMed] [Google Scholar]
- 40.Zhu W, Wang Z, Tang WHW, Hazen SL. Gut Microbe-Generated Trimethylamine N-Oxide From Dietary Choline Is Prothrombotic in Subjects. Circulation. 2017. April 25;135(17):1671–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang Z, Bergeron N, Levison BS, Li XS, Chiu S, Jia X, et al. Impact of chronic dietary red meat, white meat, or non-meat protein on trimethylamine N-oxide metabolism and renal excretion in healthy men and women. Eur Heart J. 2019. February 14;40(7):583–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, DuGar B, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011. April;472(7341):57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, et al. Intestinal microbiota metabolism of l -carnitine, a nutrient in red meat, promotes atherosclerosis. Nature Medicine. 2013. May;19(5):576–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhao Y, Yang N, Gao J, Li H, Cai W, Zhang X, et al. The Effect of Different l-Carnitine Administration Routes on the Development of Atherosclerosis in ApoE Knockout Mice. Molecular Nutrition & Food Research. 2018;62(5):1700299. [DOI] [PubMed] [Google Scholar]
- 45.Zhu W, Gregory JC, Org E, Buffa JA, Gupta N, Wang Z, et al. Gut Microbial Metabolite TMAO Enhances Platelet Hyperreactivity and Thrombosis Risk. Cell. 2016. March 24;165(1):111–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Seldin MM, Meng Y, Qi H, Zhu W, Wang Z, Hazen SL, et al. Trimethylamine N-Oxide Promotes Vascular Inflammation Through Signaling of Mitogen-Activated Protein Kinase and Nuclear Factor-κB. J Am Heart Assoc. 2016. February 22;5(2):e002767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Organ CL, Otsuka H, Bhushan S, Wang Z, Bradley J, Trivedi R, et al. Choline Diet and Its Gut Microbe-Derived Metabolite, Trimethylamine N-Oxide, Exacerbate Pressure Overload-Induced Heart Failure. Circ Heart Fail. 2015/12/23 ed. 2016. January;9(1):e002314–e002314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen K, Zheng X, Feng M, Li D, Zhang H. Gut Microbiota-Dependent Metabolite Trimethylamine N-Oxide Contributes to Cardiac Dysfunction in Western Diet-Induced Obese Mice. Front Physiol. 2017. March 21;8:139–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Heianza Y, Ma W, Manson JE, Rexrode KM, Qi L. Gut Microbiota Metabolites and Risk of Major Adverse Cardiovascular Disease Events and Death: A Systematic Review and Meta-Analysis of Prospective Studies. Journal of the American Heart Association. 6(7):e004947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Qi J, You T, Li J, Pan T, Xiang L, Han Y, et al. Circulating trimethylamine N-oxide and the risk of cardiovascular diseases: a systematic review and meta-analysis of 11 prospective cohort studies. Journal of Cellular and Molecular Medicine. 2018;22(1):185–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schiattarella GG, Sannino A, Toscano E, Giugliano G, Gargiulo G, Franzone A, et al. Gut microbe-generated metabolite trimethylamine-N-oxide as cardiovascular risk biomarker: a systematic review and dose-response meta-analysis. Eur Heart J. 2017. October 14;38(39):2948–56. [DOI] [PubMed] [Google Scholar]
- 52.Tang WHW, Wang Z, Levison BS, Koeth RA, Britt EB, Fu X, et al. Intestinal Microbial Metabolism of Phosphatidylcholine and Cardiovascular Risk. New England Journal of Medicine. 2013. April 25;368(17):1575–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tang WHW, Wang Z, Shrestha K, Borowski AG, Wu Y, Troughton RW, et al. Intestinal Microbiota-Dependent Phosphatidylcholine Metabolites, Diastolic Dysfunction, and Adverse Clinical Outcomes in Chronic Systolic Heart Failure. Journal of Cardiac Failure. 2015. February 1;21(2):91–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tang WHW, Wang Z, Fan Y, Levison B, Hazen JE, Donahue LM, et al. Prognostic Value of Elevated Levels of Intestinal Microbe-Generated Metabolite Trimethylamine-N-oxide in Patients with Heart Failure: Refining the Gut Hypothesis. J Am Coll Cardiol. 2014. November 4;64(18):1908–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schuett K, Kleber ME, Scharnagl H, Lorkowski S, März W, Niessner A, et al. Trimethylamine-N-oxide and Heart Failure With Reduced Versus Preserved Ejection Fraction. Journal of the American College of Cardiology. 2017. December 26;70(25):3202–4. [DOI] [PubMed] [Google Scholar]
- 56.Trøseid M, Ueland T, Hov JR, Svardal A, Gregersen I, Dahl CP, et al. Microbiota-dependent metabolite trimethylamine-N-oxide is associated with disease severity and survival of patients with chronic heart failure. J Intern Med. 2015. June;277(6):717–26. [DOI] [PubMed] [Google Scholar]
- 57.Suzuki T, Heaney LM, Bhandari SS, Jones DJL, Ng LL. Trimethylamine N-oxide and prognosis in acute heart failure. Heart. 2016. 01;102(11):841–8. [DOI] [PubMed] [Google Scholar]
- 58.Dodd D, Spitzer MH, Van Treuren W, Merrill BD, Hryckowian AJ, Higginbottom SK, et al. A gut bacterial pathway metabolizes aromatic amino acids into nine circulating metabolites. Nature. 2017. November 1;551(7682):648–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shimada Y, Kinoshita M, Harada K, Mizutani M, Masahata K, Kayama H, et al. Commensal Bacteria-Dependent Indole Production Enhances Epithelial Barrier Function in the Colon. PLOS ONE. 2013. November 20;8(11):e80604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bansal T, Alaniz RC, Wood TK, Jayaraman A. The bacterial signal indole increases epithelial-cell tight-junction resistance and attenuates indicators of inflammation. PNAS. 2010. January 5;107(1):228–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Venkatesh M, Mukherjee S, Wang H, Li H, Sun K, Benechet AP, et al. Symbiotic bacterial metabolites regulate gastrointestinal barrier function via the xenobiotic sensor PXR and Toll-like receptor 4. Immunity. 2014. August 21;41(2):296–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Alexeev EE, Lanis JM, Kao DJ, Campbell EL, Kelly CJ, Battista KD, et al. Microbiota-Derived Indole Metabolites Promote Human and Murine Intestinal Homeostasis through Regulation of Interleukin-10 Receptor. The American Journal of Pathology. 2018. May 1;188(5):1183–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhao Z-H, Xin F-Z, Xue Y, Hu Z, Han Y, Ma F, et al. Indole-3-propionic acid inhibits gut dysbiosis and endotoxin leakage to attenuate steatohepatitis in rats. Experimental & Molecular Medicine. 2019. September;51(9):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chimerel C, Emery E, Summers DK, Keyser U, Gribble FM, Reimann F. Bacterial Metabolite Indole Modulates Incretin Secretion from Intestinal Enteroendocrine L Cells. Cell Rep. 2014. November 13;9(4):1202–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Alexander D, Lombardi R, Rodriguez G, Mitchell MM, Marian AJ. Metabolomic distinction and insights into the pathogenesis of human primary dilated cardiomyopathy. European Journal of Clinical Investigation. 2011;41(5):527–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lekawanvijit S Role of Gut-Derived Protein-Bound Uremic Toxins in Cardiorenal Syndrome and Potential Treatment Modalities. Circ J. 2015;79(10):2088–97. [DOI] [PubMed] [Google Scholar]
- 67.Lekawanvijit S, Adrahtas A, Kelly DJ, Kompa AR, Wang BH, Krum H. Does indoxyl sulfate, a uraemic toxin, have direct effects on cardiac fibroblasts and myocytes? Eur Heart J. 2010. July 1;31(14):1771–9. [DOI] [PubMed] [Google Scholar]
- 68.Lekawanvijit S, Kompa AR, Manabe M, Wang BH, Langham RG, Nishijima F, et al. Chronic Kidney Disease-Induced Cardiac Fibrosis Is Ameliorated by Reducing Circulating Levels of a Non-Dialysable Uremic Toxin, Indoxyl Sulfate. PLOS ONE. 2012. July 19;7(7):e41281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fujii H, Nishijima F, Goto S, Sugano M, Yamato H, Kitazawa R, et al. Oral charcoal adsorbent (AST-120) prevents progression of cardiac damage in chronic kidney disease through suppression of oxidative stress. Nephrol Dial Transplant. 2009. July 1;24(7):2089–95. [DOI] [PubMed] [Google Scholar]
- 70.Shimazu S, Hirashiki A, Okumura T, Yamada T, Okamoto R, Shinoda N, et al. Association Between Indoxyl Sulfate and Cardiac Dysfunction and Prognosis in Patients With Dilated Cardiomyopathy. Circulation Journal. 2013;77(2):390–6. [DOI] [PubMed] [Google Scholar]
- 71.Yisireyili M, Saito S, Abudureyimu S, Adelibieke Y, Ng H-Y, Nishijima F, et al. Indoxyl Sulfate-Induced Activation of (Pro)renin Receptor Promotes Cell Proliferation and Tissue Factor Expression in Vascular Smooth Muscle Cells. PLOS ONE. 2014. October 24;9(10):e109268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shimizu H, Saito S, Higashiyama Y, Nishijima F, Niwa T. CREB, NF-κB, and NADPH oxidase coordinately upregulate indoxyl sulfate-induced angiotensinogen expression in proximal tubular cells. American Journal of Physiology-Cell Physiology. 2013. February 13;304(7):C685–92. [DOI] [PubMed] [Google Scholar]
- 73.Dou L, Bertrand E, Cerini C, Faure V, Sampol J, Vanholder R, et al. The uremic solutes p-cresol and indoxyl sulfate inhibit endothelial proliferation and wound repair. Kidney Int. 2004. February;65(2):442–51. [DOI] [PubMed] [Google Scholar]
- 74.Dou L, Jourde-Chiche N, Faure V, Cerini C, Berland Y, Dignat-George F, et al. The uremic solute indoxyl sulfate induces oxidative stress in endothelial cells. J Thromb Haemost. 2007. June;5(6):1302–8. [DOI] [PubMed] [Google Scholar]
- 75.Yu M, Kim YJ, Kang D-H. Indoxyl sulfate-induced endothelial dysfunction in patients with chronic kidney disease via an induction of oxidative stress. Clin J Am Soc Nephrol. 2011. January;6(1):30–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Asai M, Kumakura S, Kikuchi M. Review of the efficacy of AST-120 (KREMEZIN®) on renal function in chronic kidney disease patients. Ren Fail. 2019. February 7;41(1):47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shibahara H, Shibahara N. Cardiorenal protective effect of the oral uremic toxin absorbent AST-120 in chronic heart disease patients with moderate CKD. Journal of Nephrology. 2010. October;23(5):535–40. [PubMed] [Google Scholar]
- 78.Joubert P An in Vivo Investigation of the Negative Chronotropic Effect of Cholic Acid in the Rat. Clinical and Experimental Pharmacology and Physiology. 1978;5(1):1–8. [DOI] [PubMed] [Google Scholar]
- 79.Gazawi H, Ljubuncic P, Cogan U, Hochgraff E, Ben-Shachar D, Bomzon A. The effects of bile acids on β-adrenoceptors, fluidity, and the extent of lipid peroxidation in rat cardiac membranes. Biochemical Pharmacology. 2000. June 1;59(12):1623–8. [DOI] [PubMed] [Google Scholar]
- 80.Wang H, Chen J, Hollister K, Sowers LC, Forman BM. Endogenous Bile Acids Are Ligands for the Nuclear Receptor FXR/BAR. Molecular Cell. 1999. May 1;3(5):543–53. [DOI] [PubMed] [Google Scholar]
- 81.Kawamata Y, Fujii R, Hosoya M, Harada M, Yoshida H, Miwa M, et al. A G protein-coupled receptor responsive to bile acids. J Biol Chem. 2003. March 14;278(11):9435–40. [DOI] [PubMed] [Google Scholar]
- 82.Rosen H, Gonzalez-Cabrera PJ, Sanna MG, Brown S. Sphingosine 1-Phosphate Receptor Signaling. Annu Rev Biochem. 2009. June 1;78(1):743–68. [DOI] [PubMed] [Google Scholar]
- 83.Raufman J-P, Chen Y, Zimniak P, Cheng K. Deoxycholic Acid Conjugates Are Muscarinic Cholinergic Receptor Antagonists. PHA. 2002;65(4):215–21. [DOI] [PubMed] [Google Scholar]
- 84.Binah O, Rubinstein I, Bomzon A, Better OS. Effects of bile acids on ventricular muscle contraction and electrophysiological properties: studies in rat papillary muscle and isolated ventricular myocytes. Naunyn-Schmiedeberg’s Archives of Pharmacology. 1987. February 1;335(2):160–5. [DOI] [PubMed] [Google Scholar]
- 85.Ljubuncic P, Said O, Ehrlich Y, Meddings JB, Shaffer EA, Bomzon A. On the in vitro vasoactivity of bile acids. Br J Pharmacol. 2000. October;131(3):387–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mayerhofer CCK, Ueland T, Broch K, Vincent RP, Cross GF, Dahl CP, et al. Increased Secondary/Primary Bile Acid Ratio in Chronic Heart Failure. J Card Fail. 2017. September;23(9):666–71. [DOI] [PubMed] [Google Scholar]
- 87.von Haehling S, Schefold JC, Jankowska EA, Springer J, Vazir A, Kalra PR, et al. Ursodeoxycholic Acid in Patients With Chronic Heart Failure: A Double-Blind, Randomized, Placebo-Controlled, Crossover Trial. Journal of the American College of Cardiology. 2012. February 7;59(6):585–92. [DOI] [PubMed] [Google Scholar]
- 88.Gil-Cruz C, Perez-Shibayama C, Martin AD, Ronchi F, Borght K van der, Niederer R, et al. Microbiota-derived peptide mimics drive lethal inflammatory cardiomyopathy. Science. 2019. November 15;366(6467):881–6. [DOI] [PubMed] [Google Scholar]
- 89.Huang K, Liu Y, Tang H, Qiu M, Li C, Duan C, et al. Glabridin Prevents Doxorubicin-Induced Cardiotoxicity Through Gut Microbiota Modulation and Colonic Macrophage Polarization in Mice. Frontiers in Pharmacology. 2019;10:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Russo M, Guida F, Paparo L, Trinchese G, Aitoro R, Avagliano C, et al. The novel butyrate derivative phenylalanine-butyramide protects from doxorubicin-induced cardiotoxicity. Eur J Heart Fail. 2019;21(4):519–28. [DOI] [PubMed] [Google Scholar]
- 91.Abend A, Kehat I. Histone deacetylases as therapeutic targets — From cancer to cardiac disease. Pharmacology & Therapeutics. 2015. March 1;147:55–62. [DOI] [PubMed] [Google Scholar]
- 92.Fu Hai Ying Sanada Shoji, Takashi Matsuzaki, Yulin Liao, Keiji Okuda, Masaki Yamato, et al. Chemical Endoplasmic Reticulum Chaperone Alleviates Doxorubicin-Induced Cardiac Dysfunction. Circulation Research. 2016. March 4;118(5):798–809. [DOI] [PubMed] [Google Scholar]
- 93.Löfman I, Szummer K, Hagerman I, Dahlström U, Lund LH, Jernberg T. Prevalence and prognostic impact of kidney disease on heart failure patients. Open Heart. 2016. January 1;3(1):e000324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ronco C, Haapio M, House AA, Anavekar N, Bellomo R. Cardiorenal Syndrome. Journal of the American College of Cardiology. 2008. November 4;52(19):1527–39. [DOI] [PubMed] [Google Scholar]
- 95.Vaziri ND, Yuan J, Norris K. Role of urea in intestinal barrier dysfunction and disruption of epithelial tight junction in CKD. Am J Nephrol. 2013;37(1):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gupta N, Buffa JA, Roberts AB, Sangwan N, Skye SM, Li L, et al. Targeted Inhibition of Gut Microbial Trimethylamine N-Oxide Production Reduces Renal Tubulointerstitial Fibrosis and Functional Impairment in a Murine Model of Chronic Kidney Disease. ATVB. 2020. May;40(5):1239–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tang WHW, Wang Z Kennedy DJ, Wu Y, Buffa JA, Agatisa-Boyle B, et al. Gut Microbiota-Dependent Trimethylamine N-Oxide (TMAO) Pathway Contributes to Both Development of Renal Insufficiency and Mortality Risk in Chronic Kidney Disease. Circulation Research. 2015. January 30;116(3):448–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ingelsson E, Sundström J, Ärnlöv J, Zethelius B, Lind L. Insulin Resistance and Risk of Congestive Heart Failure. JAMA. 2005. July 20;294(3):334–41. [DOI] [PubMed] [Google Scholar]
- 99.Ärnlöv J, Lind L, Zethelius B, Andrén B, Hales CN, Vessby B, et al. Several factors associated with the insulin resistance syndrome are predictors of left ventricular systolic dysfunction in a male population after 20 years of follow-up. American Heart Journal. 2001. October 1;142(4):720–4. [DOI] [PubMed] [Google Scholar]
- 100.Witteles RM, Tang WHW, Jamali AH, Chu JW, Reaven GM, Fowler MB. Insulin resistance in idiopathic dilated cardiomyopathy: A possible etiologic link. Journal of the American College of Cardiology. 2004. July 7;44(1):78–81. [DOI] [PubMed] [Google Scholar]
- 101.Suskin N, McKelvie RS, Burns RJ, Latini R, Pericak D, Probstfield J, et al. Glucose and insulin abnormalities relate to functional capacity in patients with congestive heart failure. Eur Heart J. 2000. August 1;21(16):1368–75. [DOI] [PubMed] [Google Scholar]
- 102.ALZadjali MA, Godfrey V, Khan F, Choy A, Doney AS, Wong AK, et al. Insulin Resistance Is Highly Prevalent and Is Associated With Reduced Exercise Tolerance in Nondiabetic Patients With Heart Failure. Journal of the American College of Cardiology. 2009. March 3;53(9):747–53. [DOI] [PubMed] [Google Scholar]
- 103.Doehner W, Rauchhaus M, Ponikowski P, Godsland IF, von Haehling S, Okonko DO, et al. Impaired Insulin Sensitivity as an Independent Risk Factor for Mortality in Patients With Stable Chronic Heart Failure. Journal of the American College of Cardiology. 2005. September 20;46(6):1019–26. [DOI] [PubMed] [Google Scholar]
- 104.Paolisso G, Tagliamonte MR, Rizzo MR, Gambardella A, Gualdiero P, Lama D, et al. Prognostic importance of insulin-mediated glucose uptake in aged patients with congestive heart failure secondary to mitral and/or aortic valve disease. American Journal of Cardiology. 1999. May 1;83(9):1338–44. [DOI] [PubMed] [Google Scholar]
- 105.Witteles RM, Fowler MB. Insulin-Resistant Cardiomyopathy: Clinical Evidence, Mechanisms, and Treatment Options. Journal of the American College of Cardiology. 2008. January 15;51(2):93–102. [DOI] [PubMed] [Google Scholar]
- 106.Tenenbaum A, Fisman EZ. Impaired Glucose Metabolism in Patients with Heart Failure. Am J Cordiovosc Drugs. 2004. September 1;4(5):269–80. [DOI] [PubMed] [Google Scholar]
- 107.Kostis JB, Sanders M. The Association of Heart Failure With Insulin Resistance and the Development of Type 2 Diabetes. Am J Hypertens. 2005. May 1;18(5):731–7. [DOI] [PubMed] [Google Scholar]
- 108.Bouter KE, Raalte DH van, Groen AK, Nieuwdorp M. Role of the Gut Microbiome in the Pathogenesis of Obesity and Obesity-Related Metabolic Dysfunction. Gastroenterology. 2017. May 1;152(7):1671–8. [DOI] [PubMed] [Google Scholar]
- 109.Karlsson FH, Tremaroli V, Nookaew I, Bergström G, Behre CJ, Fagerberg B, et al. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature. 2013. June;498(7452):99–103. [DOI] [PubMed] [Google Scholar]
- 110.Vrieze A, Nood EV, Holleman F, Salojärvi J, Kootte RS, Bartelsman JFWM, et al. Transfer of Intestinal Microbiota From Lean Donors Increases Insulin Sensitivity in Individuals With Metabolic Syndrome. Gastroenterology. 2012. October 1;143(4):913–916.e7. [DOI] [PubMed] [Google Scholar]
- 111.Cheng M-L, Wang C-H, Shiao M-S, Liu M-H, Huang Y-Y, Huang C-Y, et al. Metabolic Disturbances Identified in Plasma Are Associated With Outcomes in Patients With Heart Failure: Diagnostic and Prognostic Value of Metabolomics. Journal of the American College of Cardiology. 2015. April 21;65(15):1509–20. [DOI] [PubMed] [Google Scholar]
- 112.Hunter WG, Kelly JP, McGarrah RW, Kraus WE, Shah SH. Metabolic Dysfunction in Heart Failure: Diagnostic, Prognostic, and Pathophysiologic Insights From Metabolomic Profiling. Curr Heart Fail Rep. 2016. June 1;13(3):119–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Newgard CB, An J, Bain JR, Muehlbauer MJ, Stevens RD, Lien LF, et al. A Branched-Chain Amino Acid-Related Metabolic Signature that Differentiates Obese and Lean Humans and Contributes to Insulin Resistance. Cell Metab. 2009. April;9(4):311–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Palmer ND, Stevens RD, Antinozzi PA, Anderson A, Bergman RN, Wagenknecht LE, et al. Metabolomic Profile Associated With Insulin Resistance and Conversion to Diabetes in the Insulin Resistance Atherosclerosis Study. J Clin Endocrinol Metab. 2015. March;100(3):E463–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Pedersen HK, Gudmundsdottir V, Nielsen HB, Hyotylainen T, Nielsen T, Jensen BAH, et al. Human gut microbes impact host serum metabolome and insulin sensitivity. Nature. 2016. July;535(7612):376–81. [DOI] [PubMed] [Google Scholar]
- 116.Sanna S, van Zuydam NR, Mahajan A, Kurilshikov A, Vich Vila A, Võsa U, et al. Causal relationships among the gut microbiome, short-chain fatty acids and metabolic diseases. Nature Genetics. 2019. April;51(4):600–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tirosh A, Calay ES, Tuncman G, Claiborn KC, Inouye KE, Eguchi K, et al. The short-chain fatty acid propionate increases glucagon and FABP4 production, impairing insulin action in mice and humans. Sci Transl Med. 2019. April 24;11(489). [DOI] [PubMed] [Google Scholar]
- 118.Gao X, Liu X, Xu J, Xue C, Xue Y, Wang Y. Dietary trimethylamine N-oxide exacerbates impaired glucose tolerance in mice fed a high fat diet. Journal of Bioscience and Bioengineering. 2014. October 1;118(4):476–81. [DOI] [PubMed] [Google Scholar]
- 119.Shan Z, Sun T, Huang H, Chen S, Chen L, Luo C, et al. Association between microbiota-dependent metabolite trimethylamine-N-oxide and type 2 diabetes. Am J Clin Nutr. 2017. September 1;106(3):888–94. [DOI] [PubMed] [Google Scholar]
- 120.Haeusler RA, Astiarraga B, Camastra S, Accili D, Ferrannini E. Human Insulin Resistance Is Associated With Increased Plasma Levels of 12α-Hydroxylated Bile Acids. Diabetes. 2013. December 1;62(12):4184–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Legry V, Francque S, Haas JT, Verrijken A, Caron S, Chávez-Talavera O, et al. Bile Acid Alterations Are Associated With Insulin Resistance, but Not With NASH, in Obese Subjects. J Clin Endocrinol Metab. 2017. 01;102(10):3783–94. [DOI] [PubMed] [Google Scholar]
- 122.Ahmad TR, Haeusler RA. Bile acids in glucose metabolism and insulin signalling — mechanisms and research needs. Nature Reviews Endocrinology. 2019. December;15(12):701–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sundaram V, Fang JC. Gastrointestinal and Liver Issues in Heart Failure. Circulation. 2016. April 26;133(17):1696–703. [DOI] [PubMed] [Google Scholar]
- 124.Genton L, Mareschal J, Charretier Y, Lazarevic V, Bindels LB, Schrenzel J. Targeting the Gut Microbiota to Treat Cachexia. Frontiers in Cellular and Infection Microbiology. 2019;9:305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Emami A, Saitoh M, Valentova M, Sandek A, Evertz R, Ebner N, et al. Comparison of sarcopenia and cachexia in men with chronic heart failure: results from the Studies Investigating Co-morbidities Aggravating Heart Failure (SICA-HF). European Journal of Heart Failure. 2018;20(11):1580–7. [DOI] [PubMed] [Google Scholar]
- 126.Zhou J, Liu B, Liang C, Li Y, Song Y-H. Cytokine Signaling in Skeletal Muscle Wasting. Trends in Endocrinology & Metabolism. 2016. May 1;27(5):335–47. [DOI] [PubMed] [Google Scholar]
- 127.Garcia-Lafuente A, Antolin M, Guarner F, Crespo E, Malagelada J. Modulation of colonic barrier function by the composition of the commensal flora in the rat. Gut. 2001. April;48(4):503–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hiippala K, Jouhten H, Ronkainen A, Hartikainen A, Kainulainen V, Jalanka J, et al. The Potential of Gut Commensals in Reinforcing Intestinal Barrier Function and Alleviating Inflammation. Nutrients. 2018. August;10(8):988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zimmermann M, Zimmermann-Kogadeeva M, Wegmann R, Goodman AL. Mapping human microbiome drug metabolism by gut bacteria and their genes. Nature. 2019. June;570(7762):462–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Maier L, Pruteanu M, Kuhn M, Zeller G, Telzerow A, Anderson EE, et al. Extensive impact of non-antibiotic drugs on human gut bacteria. Nature. 2018. March;555(7698):623–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Saha Butler VP, Neu HC, Lindenbaum J Digoxin-inactivating bacteria: identification in human gut flora. Science. 1983. April 15;220(4594):325–7. [DOI] [PubMed] [Google Scholar]
- 132.Haiser HJ, Gootenberg DB, Chatman K, Sirasani G, Balskus EP, Turnbaugh PJ. Predicting and manipulating cardiac drug inactivation by the human gut bacterium Eggerthella lenta. Science. 2013. July 19;341(6143):295–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yoo HH, Kim IS, Yoo D-H, Kim D-H. Effects of orally administered antibiotics on the bioavailability of amlodipine: gut microbiota-mediated drug interaction. Journal of Hypertension. 2016. January;34(1):156–162. [DOI] [PubMed] [Google Scholar]
- 134.Estruch R, Ros E, Salas-Salvadó J, Covas M-I, Corella D, Arós F, et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N Engl J Med. 2018. June 13;378(25):e34. [DOI] [PubMed] [Google Scholar]
- 135.Tektonidis TG, Åkesson A, Gigante B, Wolk A, Larsson SC. A Mediterranean diet and risk of myocardial infarction, heart failure and stroke: A population-based cohort study. Atherosclerosis. 2015. November 1;243(1):93–8. [DOI] [PubMed] [Google Scholar]
- 136.Sanches Machado d’Almeida K, Ronchi Spillere S, Zuchinali P, Corrêa Souza G. Mediterranean Diet and Other Dietary Patterns in Primary Prevention of Heart Failure and Changes in Cardiac Function Markers: A Systematic Review. Nutrients. 2018. January 10;10(1):58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wang Z, Roberts AB, Buffa JA, Levison BS, Zhu W, Org E, et al. Non-lethal Inhibition of Gut Microbial Trimethylamine Production for the Treatment of Atherosclerosis. Cell. 2015. December 17;163(7):1585–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Roberts AB, Gu X, Buffa JA, Hurd AG, Wang Z, Zhu W, et al. Development of a gut microbe–targeted nonlethal therapeutic to inhibit thrombosis potential. Nature Medicine. 2018. September;24(9):1407–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lara KM, Levitan EB, Gutierrez OM, Shikany JM, Safford MM, Judd SE, et al. Dietary Patterns and Incident Heart Failure in U.S. Adults Without Known Coronary Disease. J Am Coll Cardiol. 2019. April 30;73(16):2036–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Campos CL, Wood A, Burke GL, Bahrami H, Bertoni AG. Dietary Approaches to Stop Hypertension Diet Concordance and Incident Heart Failure: The Multi-Ethnic Study of Atherosclerosis. American Journal of Preventive Medicine. 2019. June 1;56(6):819–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Levitan EB, Wolk A, Mittleman MA. Consistency With the DASH Diet and Incidence of Heart Failure. Arch Intern Med. 2009. May 11;169(9):851–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Levitan Emily B, Lewis Cora E, Tinker Lesley F, Eaton Charles B, Ahmed Ali, Manson JoAnn E., et al. Mediterranean and DASH Diet Scores and Mortality in Women With Heart Failure. Circulation: Heart Failure. 2013. November 1;6(6):1116–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Miró Ò, Estruch R, Martín-Sánchez FJ, Gil V, Jacob J, Herrero-Puente P, et al. Adherence to Mediterranean Diet and All-Cause Mortality After an Episode of Acute Heart Failure: Results of the MEDIT-AHF Study. JACC: Heart Failure. 2018. January 1;6(1):52–62. [DOI] [PubMed] [Google Scholar]
- 144.Tuttolomondo A, Di Raimondo D, Casuccio A, Velardo M, Salamone G, Cataldi M, et al. Mediterranean diet adherence and congestive heart failure: Relationship with clinical severity and ischemic pathogenesis. Nutrition. 2020. February 1;70:110584. [DOI] [PubMed] [Google Scholar]
- 145.Desai MS, Seekatz AM, Koropatkin NM, Kamada N, Hickey CA, Wolter M, et al. A dietary fiber-deprived gut microbiota degrades the colonic mucus barrier and enhances pathogen susceptibility. Cell. 2016. November 17;167(5):1339–1353.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Chassaing B, Raja SM, Lewis JD, Srinivasan S, Gewirtz AT. Colonic Microbiota Encroachment Correlates With Dysglycemia in Humans. Cellular and Molecular Gastroenterology and Hepatology. 2017. September 1;4(2):205–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Nagpal R, Shively CA, Register TC, Craft S, Yadav H. Gut microbiome-Mediterranean diet interactions in improving host health. F1000Res. 2019. May 21;8:699–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wilck N, Matus MG, Kearney SM, Olesen SW, Forslund K, Bartolomaeus H, et al. Salt-responsive gut commensal modulates T H 17 axis and disease. Nature. 2017. November;551(7682):585–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Bier A, Braun T, Khasbab R, Di Segni A, Grossman E, Haberman Y, et al. A High Salt Diet Modulates the Gut Microbiota and Short Chain Fatty Acids Production in a Salt-Sensitive Hypertension Rat Model. Nutrients. 2018. August 23;10(9):1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Gan XT, Ettinger G, Huang CX, Burton JP, Haist JV, Rajapurohitam V, et al. Probiotic Administration Attenuates Myocardial Hypertrophy and Heart Failure After Myocardial Infarction in the Rat. Circulation: Heart Failure. 2014. May 1;7(3):491–9. [DOI] [PubMed] [Google Scholar]
- 151.Lam V, Su J, Koprowski S, Hsu A, Tweddell JS, Rafiee P, et al. Intestinal microbiota determine severity of myocardial infarction in rats. The FASEB Journal. 2012;26(4):1727–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Costanza AC, Moscavitch SD, Neto HCCF, Mesquita ET. Probiotic therapy with Saccharomyces boulardii for heart failure patients: A randomized, double-blind, placebo-controlled pilot trial. International Journal of Cardiology. 2015. January 20;179:348–50. [DOI] [PubMed] [Google Scholar]
- 153.Mayerhofer CCK, Awoyemi AO, Moscavitch SD, Lappegård KT, Hov JR, Aukrust P, et al. Design of the GutHeart—targeting gut microbiota to treat heart failure—trial: a Phase II, randomized clinical trial. ESC Heart Failure. 2018;5(5):977–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Gullestad L GutHeart: Targeting Gut Microbiota to Treat Heart Failure [Internet]. clinicaltrials.gov; 2019. March [cited 2020 Aug 27]. Report No.: NCT02637167. Available from: https://clinicaltrials.gov/ct2/show/NCT02637167
- 155.Wang Z, Roberts AB, Buffa JA, Levison BS, Zhu W, Org E, et al. Non-lethal Inhibition of Gut Microbial Trimethylamine Production for the Treatment of Atherosclerosis. Cell. 2015. December 17;163(7):1585–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Roberts AB, Gu X, Buffa JA, Hurd AG, Wang Z, Zhu W, et al. Development of a gut microbe–targeted nonlethal therapeutic to inhibit thrombosis potential. Nature Medicine. 2018. September;24(9):1407–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Organ CL, Li Z, Sharp TE, Polhemus DJ, Gupta N, Goodchild TT, et al. Nonlethal Inhibition of Gut Microbial Trimethylamine N-oxide Production Improves Cardiac Function and Remodeling in a Murine Model of Heart Failure. Journal of the American Heart Association. 2020. May 18;9(10):e016223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.McDonald LC, Gerding DN, Johnson S, Bakken JS, Carroll KC, Coffin SE, et al. Clinical Practice Guidelines for Clostridium difficile Infection in Adults and Children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis. 2018. March 19;66(7):987–94. [DOI] [PubMed] [Google Scholar]
- 159.Kootte RS, Levin E, Salojärvi J, Smits LP, Hartstra AV, Udayappan SD, et al. Improvement of Insulin Sensitivity after Lean Donor Feces in Metabolic Syndrome Is Driven by Baseline Intestinal Microbiota Composition. Cell Metabolism. 2017. October 3;26(4):611–619.e6. [DOI] [PubMed] [Google Scholar]
- 160.DeFilipp Z, Bloom PP, Torres Soto M, Mansour MK, Sater MRA, Huntley MH, et al. Drug-Resistant E. coli Bacteremia Transmitted by Fecal Microbiota Transplant. New England Journal of Medicine. 2019. November 21;381(21):2043–50. [DOI] [PubMed] [Google Scholar]