Abstract
Some older adults with insomnia experience sleep discrepancy, often characterized by greater subjective sleep difficulties and shorter subjective sleep duration than the estimates derived from objective measures. The present study examined whether a brief behavioral therapy for insomnia (BBTi) is efficacious for reducing sleep discrepancy in older adults. This study is a secondary analysis of a randomized controlled trial of BBTi for community dwelling older adults with chronic insomnia (N=62). Thirty-two participants received BBTi, delivered in four individual face-to-face sessions. Thirty received the self-monitoring control (SMC). They all completed daily sleep diaries and wore an actigraph from baseline to posttreatment, and for two weeks at 3-month follow-up. Sleep discrepancy was calculated by subtracting diary from actigraphy estimates of sleep onset latency (SOL), wake after sleep onset (WASO), and total sleep time (TST). Mixed modeling was used to analyze data. SOL discrepancy decreased significantly in BBTi participants compared to SMC participants. The decreases in SOL discrepancy were explained by changes in diary-assessed SOL and subjective sleep quality but not changes in actigraphy-assessed SOL. Although WASO discrepancy and TST discrepancy decreased from baseline to posttreatment and follow-up, the Time by Group interaction effects were not significant indicating that BBTi participants did not experience greater reductions in WASO discrepancy and TST discrepancy than SMC participants. In conclusion, BBTi is efficacious for reducing SOL discrepancy in older adults with chronic insomnia.
Keywords: Sleep discrepancy, sleep perception, insomnia, older adults, behavioral therapy for insomnia
Previous studies have shown that subjective experience of sleep states is not always corroborated by objective measures of sleep (A. G. Harvey & Tang, 2012; Rezaie, Fobian, McCall, & Khazaie, 2018). Sleep discrepancy, defined as the difference between subjective and objective sleep parameters, is experienced by some individuals with insomnia and is characterized by greater subjective sleep difficulties and shorter sleep duration than polysomnography and actigraphy estimates of the same parameters (Kay, Buysse, Germain, Hall, & Monk, 2015; McCrae et al., 2005; Rezaie et al., 2018; Williams, Kay, Rowe, & McCrae, 2013). Rather than a misperception of sleep states, sleep discrepancy is considered a unique abnormal experience during the transition between sleep and wakefulness in some individuals with insomnia (Bastien et al., 2014; McCrae et al., 2005; M. L. Perlis, Giles, Mendelson, Bootzin, & Wyatt, 1997). Sleep discrepancy is an important clinical outcome in the treatment of insomnia because it perpetuates chronic insomnia (A. G. Harvey & Tang, 2012; M. L. Perlis et al., 1997). For instance, sleep discrepancy may lead to worsening maladaptive behaviors, such as extending time in bed and staying in bed while awake, which contribute to conditioned arousal and maintain sleep difficulties (M. L. Perlis et al., 1997). Sleep discrepancy may also be an indicator of sustaining neurobiological hyperarousal that perpetuates the experience of insomnia (Riemann et al., 2010). A reduction in sleep discrepancy has been observed following cognitive behavioral therapy for insomnia (CBTi) (Dzierzewski et al., 2019; Kay et al., 2015; Lund, Rybarczyk, Perrin, Leszczyszyn, & Stepanski, 2013). The present study contributes to the existing literature by investigating the changes in sleep discrepancy during brief behavioral therapy for insomnia (BBTi), which has higher potential for widespread use than CBTi due to its greater cost-effectiveness and ease of being disseminated in primary-care settings.
Chronic insomnia is diagnosed by subjective complaints of difficulty initiating sleep, difficulty maintaining sleep, or early waking for at least three nights per week for at least 3 months and are accompanied by significant distress or impaired functioning (American Psychiatric Association, 2013; Edinger et al., 2006). The hyperarousal model of chronic insomnia postulates that, following the initiation of an insomnia episode, individuals engage in maladaptive cognitive and behavioral compensatory strategies including increased worry and concern about sleep, attentional biases towards insomnia-related consequences, behavioral changes leading to increased time in bed during wakefulness, and increased consumption of stimulants and reduction in daytime activities to counteract daytime consequences; these strategies lead to heightened cognitive and physiological arousal, sustained by neurobiological alterations, leading to difficulties falling and staying asleep (M. L. Perlis et al., 1997; Riemann et al., 2010). Sleep discrepancy is the consequence of increased sensory and information processing during the transition between wakefulness and sleep, resulting from heightened physiological and cognitive arousal (A. G. Harvey & Tang, 2012; M. L. Perlis et al., 1997). Indeed, experimentally induced pre-sleep cognitive and physiological arousal were found to increase sleep discrepancy (Tang & Harvey, 2004). Sleep discrepancy was also found to be associated with increased neuroactivity in the prefrontal-parietal cortex during sleep onset (Hsiao et al., 2018).
Sleep discrepancy has been frequently documented in older adults with subjective complaints of sleep problems or insomnia (Dinapoli et al., 2017; Kay et al., 2015; O’Donnell et al., 2009; Williams et al., 2013). It has also been shown to be positively associated with age in a sample of patients with depression (Tsuchiyama, Nagayama, Kudo, Kojima, & Yamada, 2003). Changes in sleep architecture is a normal part of aging. Older adults’ sleep is characterized by longer duration of light sleep (N1 and N2 sleep stages) and shorter duration of deep sleep (Ohayon, Carskadon, Guilleminault, & Vitiello, 2003). These changes may predispose older adults to increased sensory and information processing during the transition between sleep and wakefulness and elevate the experience of sleep discrepancy.
CBTi is the first-line treatment for chronic insomnia (Qaseem et al., 2016) and has well-established effectiveness for treating late-life insomnia (Morin, Colecchi, Stone, Sood, & Brink, 1999). Sleep discrepancy in older adults has also been found to decrease after six to eight sessions of CBTi (Dzierzewski et al., 2019; Kay et al., 2015; Lund et al., 2013). Lund et al. found that the reduction in sleep discrepancy in older adults was associated with the reduction in the percentage of stage N1 sleep, suggesting that subjective sleep estimation improved along with improved objective sleep quality. Likewise, Dzierzewski et al. also found that improved subjective sleep quality during CBTi predicted reduced sleep discrepancy. Although CBTi is a recommended treatment for insomnia, limited access to trained CBTi providers remains a key barrier in its implementation (Koffel, Bramoweth, & Ulmer, 2018).
BBTi, which includes only the behavioral components of CBTi, has been found to be equally efficacious as CBTi for improving insomnia in older adults (Buysse et al., 2011; McCrae et al., 2018). With its shorter length, fewer in-person visits, and the exclusion of the cognitive techniques that are often acquired through specialty training in clinical psychology, BBTi can be implemented with greater cost-effectiveness and ease in primary medicine settings by nurses and other mental health practicioners who do not have specialty training (Buysse et al., 2011). The behavioral components in BBTi include stimulus control, i.e., breaking the association between non-sleep activity and the sleeping environment in order to reduce conditioned arousal, sleep restriction, i.e., reducing sleep opportunity in order to improve sleep drive, sleep hygiene, i.e., reducing sleep-interfering habits, and relaxation, i.e., reducing arousal (Lichstein & Morin, 2000; McCrae et al., 2018). Although dysfunctional thoughts about sleep are one of the maintaining mechanisms underlying hyperarousal and insomnia, directly addressing these thoughts using cognitive techniques may not be necessary in reducing sleep discrepancy for two reasons. First, stimulus control and relaxation are also aimed at reducing arousal and might be sufficient for alleviating hyperarousal. Second, based on the cognitive behavioral model, changing behaviors can bring about cognitive changes just as changing cognitions can bring about behavioral changes (Brewin, 1989). By learning adaptive sleep habits such as reducing sleep opportunity as opposed to its lengthening, one could experience improved sleep drive and improved sleep quality, which counteract the dysfunctional beliefs such as “I need to go to bed earlier because it takes me a long time to fall asleep” or “At least I am getting some rest when I am lying in bed awake.” Hence, we hypothesized BBTi could effectively reduce sleep discrepancy despite not directly addressing dysfunctional beliefs about sleep.
This study is a secondary analysis of a randomized controlled trial (RCT) of the efficacy of BBTi for a sample of community dwelling adults with chronic insomnia (McCrae et al., 2018). BBTi, compared to a self-monitoring control group (SMC), was found to be efficacious for improving insomnia symptoms in this trial. In this study, we examined whether BBTi participants experienced greater reductions of sleep discrepancy compared to SMC participants. It was hypothesized that sleep discrepancy would decrease in individuals who received BBTi compared to SMC.
Methods
Participants
A total of 62 community dwelling older adults (mean age = 69.45 [7.71], 67.74% female, 82.26% white, 2% African American, 2% Asian, and 3% multiracial) who met diagnostic criteria for chronic insomnia (American Psychiatric Association, 2013) participated in this study. Those who had other sleep disorders (e.g., sleep apnea and periodic limb movement disorder), severe psychiatric illnesses (e.g., schizophrenia and substance abuse), neurological disorders (e.g., dementia), severe depressive symptomatology, and significant cognitive impairment were excluded. Among all sample characteristics including demographics and clinical characteristics, only insomnia duration was significantly different between BBTi and SMC participants (McCrae et al., 2018). Controlling for insomnia duration did not have any impact on the results of the present study. Details on recruitment, inclusion and exclusion criteria, randomization, and CONSORT diagram were published elsewhere (McCrae et al., 2018).
This study is registered on ClinicalTrials.gov as NCT02967185. Participants were randomized with 32 in the treatment condition (22 female,10 male; mean age 67.97, SD 5.97) and 30 in SMC (20 female, 10 male; mean age 71.03, SD 9.06). Randomization was conducted using computer-generated block randomization. Research staff involved in recruitment and assessment were blinded to the participant assignment but interventionist and participants were not. Sample size was determined by an a priori power analysis in which we estimated moderate effects (ES = .5) for the group by time interactions for the primary outcomes presented in the trial main paper. Institutional review board approval was obtained prior to participant recruitment.
Treatment
BBTi was delivered as a manualized treatment and consisted of 4 weekly, 1-hour face-to-face individual sessions. The treatment included instructions for four techniques: sleep hygiene, sleep restriction, stimulus control, and relaxation (Lichstein & Morin, 2000; McCrae et al., 2018). The instructions are designed to: (a) promote good sleep habits, (b) restrict the use of the bed and bedroom to sleep and sleep-conducive activities, (c) modify bed and wake times to better match the participants’ sleep needs, and (d) promote relaxation. Daily home practice of these techniques was encouraged. All treatment was provided for the participants at no cost. Participants maintained logs of their home practice sessions and recorded their daily sleep habits throughout treatment. During each session, the therapist reviewed these logs and discussed progress with the participant. When problems were encountered, the therapist and participant worked together to resolve them. In the final session, participants received a chart documenting their progress in therapy, and steps for maintaining continued use of the techniques taught during therapy were discussed. The therapists were three predoctoral students in an American Psychological Association (APA) approved counseling psychology program. These therapists were trained and supervised by author CSM, a licensed clinical psychologist certified in behavioral sleep medicine by the American Board of Sleep Medicine. To ensure treatment integrity, CSM held weekly group supervision meetings with the therapists. Additionally, treatment integrity was ensured by audio taping sessions, half of which were reviewed by therapists, and a quarter of which were reviewed by CSM. Participants assigned to the SMC also met with a therapist for one hour weekly for 4 weeks and participated in social conversations with the therapist that were not related to the BBTi intervention details or sleep. They also completed the same assessments (i.e., sleep diary, actigraphy) as the BBTi group.
Measures
Sleep diaries.
Participants were instructed to complete a sleep diary upon rising each morning throughout the study (10 weeks total = 2 weeks at baseline, 4 weeks of treatment/control, 2 weeks at post-treatment, and 2 weeks at 3-month follow-up). The sleep diary provided the following variables: (1) sleep onset latency (SOL)-time from initial lights-out until sleep onset; (2) wake time after sleep onset (WASO)-time spent awake after initial sleep onset until last awakening; (3) total sleep time (TST)-computed by subtracting total wake time from time spent in bed, and, (4) subjective sleep quality-participants rated their sleep quality on a 5-point scale from 0-poor to 4-very good.
Actigraphy.
Participants wore an Actiwatch-L (ACTL) with an integral ambient light sensor (Mini Mitter Co., Inc.) on their non-dominant wrists continuously throughout the study (10 weeks). The ACTL monitored ambient light exposure and gross motor activity and contains an omnidirectional piezoelectric accelerometer with sensitivity of 0.01g-force or greater. The sensors of the ACTL were sampled 32 times per second and record peak values for each second. These peak values were then summed into 30-second “activity” counts. These activity counts were downloaded to a personal computer and analyzed using Actiware-Sleep v. 3.3. The sleep and wake scoring algorithm used for the piezoelectric accelerometer has been validated against polysomnography (Weiss, Johnson, Berger, & Redline, 2010). Bedtime and time out of bed in the morning were based on sleep diary entries as recommended in the software manual. Actiware-Sleep determined sleep start automatically by searching for the first 10 minutes during which no more than one epoch was scored as wake. Likewise, sleep end was the last 10 minutes during which no more than one epoch was scored as wake. The software provides three default sensitivity settings (high, medium, low). This study utilized medium sensitivity, which sets the threshold at 40 activity counts. If the total activity for an epoch was ≥ 40, it was scored as wake. If the total activity was ≤ 40, the final activity count for the epoch was based on the level of activity in the surrounding 2 minutes. The sleep parameters derived from actigraphy included SOL, WASO, and TST.
Sleep discrepancy variables.
Similar to prior studies on sleep discrepancy (Dzierzewski et al., 2019; Williams et al., 2013), sleep discrepancy for each day was calculated by subtracting actigraphy-assessed sleep parameters from diary-assessed sleep parameters for each night. Three discrepancy variables were created including SOL discrepancy (diary – actigraphy SOL), WASO discrepancy (diary – actigraphy WASO), and TST discrepancy (diary – actigraphy TST). The weekly averages of daily sleep discrepancy were then submitted to the subsequent analyses.
Data Analysis
All analysis was conducted in R Version 1.1.463 using the lmer package. Mixed models were used to examine changes of weekly sleep discrepancy variables from baseline to posttreatment (2 weeks of baseline, 4 weeks of BBTi/SMC, 2 weeks of posttreatment) and from posttreatment to 3-month follow-up (2 weeks). The dependent variables were SOL discrepancy, WASO discrepancy, and TST discrepancy. The independent variables were Time (Week), Group (BBTi vs SMC), and the Time by Group Interaction. For instance, the mixed model equation for the model of SOL discrepancy is as follows:
SOL discrepancy for individual j at time i was regressed on the intercept (γ00), initial levels of SOL discrepancy for each group (γ01), the slope of change across time (γ10), the cross-level Time by Group interaction (γ11), the random effects of the intercept and Time (u0j + u1j), and the individual-level residual variance (rij). Effect sizes of the Time by Group interaction were calculated as the proportion of variance in the random effect of Time explained by Group, i.e., how much between-individual variability in the changes in SOL discrepancy across time was explained by the group assignment. If there was a significant reduction in sleep discrepancy, time-variant diary-assessed and actigraphy-assessed sleep parameters and subjective sleep quality were subsequently included in the model to evaluate whether the reduction in sleep discrepancy was explained by a reduction in diary- or actigraphy-assessed sleep parameters or subjective sleep quality.
All randomized participants were included in analyses following an intent-to-treat approach (McCrae et al., 2018). Three participants had missing data on all sleep discrepancy variables due to missing actigraphy data. A total of 526 observations from 59 participants with data on sleep discrepancy variables were analyzed. Missing data on independent variables were handled using the full information maximum likelihood estimation. Bonferroni corrections of p-values were be used to adjust for running three separate models, i.e., p-value of .016 or smaller would indicate statistical significance.
Results
Table 1 presents the means and standard deviations of sleep discrepancy of participants in the BBTi and SMC group at baseline, post-treatment, and follow-up. At baseline, both BBTi and SMC participants experienced greater subjective sleep difficulties than objective estimates, i.e., greater diary estimates of SOL and WASO and smaller estimates of TST compared to actigraphy estimates. There were significant differences in WASO and TST discrepancies between BBTi and SMC participants at baseline (see Table 1).
Table 1.
Means and Standard Deviations of Sleep Discrepancy
| Baseline | Post-treatment | Follow-up | |||||||
|---|---|---|---|---|---|---|---|---|---|
| BBTi | SMC | t- value (p- value) |
BBTi | SMC | t- value (p- value) |
BBTi | SMC | t- value (p- value) |
|
| SOL Discrepancy | 32.38 (32.38) | 21.31 (43.48) | .76 (.28) | 11.75 (20.67) | 15.91 (42.46) | .51 (.61) | 7.57 (28.85) | 21.94 (83.83) | .87 (.39) |
| WASO Discrepancy | 26.30 (37.16) | 12.36 (27.10) | −1.60 (.04) | 8.83 (32.56) | 1.75 (27.32) | −.89 (.38) | 6.52 (36.38) | .18 (32.78) | −.68 (.50) |
| TST Discrepancy | −46.91 (49.83) | −13.57 (71.29) | 2.04 (.05) | 4.22 (141.54) | 12.11 (69.24) | .27 (.79) | 17.08 (142.78) | 2.07 (81.07) | −.47 (.64) |
Note. BBTi – Brief behavioral therapy of Insomnia; SMC – Self-monitoring control; SOL – Sleep onset latency; WASO – Wake after sleep onset; TST – Total sleep time. Values are in minutes. Positive values indicate that diary-assessed parameters are greater than actigraphy-assessed parameters. Negative values indicate that actigraphy-assessed parameters are greater than diary-assessed parameters.
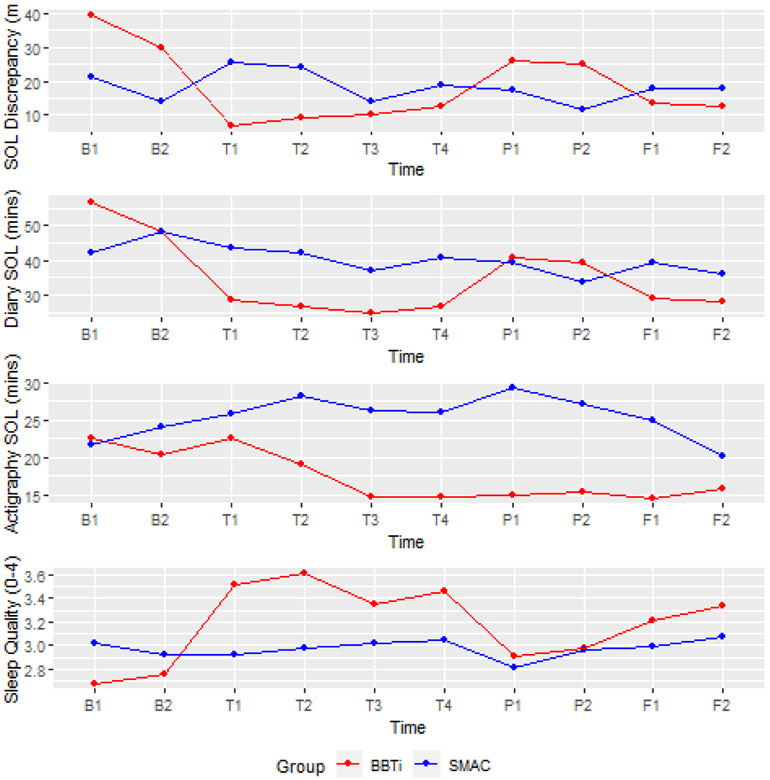
Although there were reductions in SOL, WASO, and TST discrepancies from baseline to posttreatment and follow-up, only SOL discrepancy significantly decreased in BBTi compared to SMC (see Table 2). The Time by Group interaction effect was significant (γ11 = −3.58, p= .004). The interaction effect explained 15% of the between-individual variability in the changes in SOL discrepancy across time. As shown in the top panel of Figure 1, SOL discrepancy reduced from 40 mins to below 10 mins in BBTi participants, whereas SOL discrepancy stayed about the same in SMC participants.
Table 2.
Fixed Effects of the Mixed Effects Models for SOL Discrepancy
| Model 1 | Model 2 – controlling for diary-assessed SOL |
Model 3- controlled for actigraphy- assessed SOL |
Model 4 – controlling for subjective sleep quality |
|||||
|---|---|---|---|---|---|---|---|---|
| γ | p | γ | p | γ | p | γ | p | |
| Intercept | 16.08 | .002 | −16.93 | <.001 | 29.05 | <.001 | 39.73 | <.001 |
| Time (assessment point) | .24 | .794 | .20 | .672 | .27 | .760 | .31 | .755 |
| Group (BBTi vs SMAC) | 19.59 | .007 | 9.01 | .034 | 15.88 | .049 | 16.71 | .017 |
| Time by Group | −3.58 | .006 | −.90 | .167 | −3.58 | .004 | −2.79 | .042 |
| SOL-diary | .84 | <.001 | ||||||
| SOL-actigraphy | −.54 | <.001 | ||||||
| Subjective sleep quality | −8.01 | <.001 | ||||||
Note. SOL – Sleep onset latency; BBTi – Brief behavioral therapy for insomnia. SMC – Self-monitoring control.
Figure 1.
Top panel: Changes in sleep onset latency (SOL) discrepancy. Second panel: Changes in diary-assessed SOL. Third panel: Changes in actigraphy-assessed SOL. Bottom panel: Changes in subjective sleep quality. X-axis labels denote assessment time points; B—baseline, T—treatment, P – posttreatment, F – follow up.
The reduction in SOL discrepancy appeared to be attributable to the reduction in diary-assessed SOL but not actigraphy-assessed SOL. When diary-assessed SOL was included in the model, the reduction in SOL discrepancy became non-significant; however, the reduction in SOL discrepancy remained significant after controlling for changes in actigraphy-assessed SOL (see Table 2). When subjective sleep quality was included in the model, the reduction in SOL discrepancy became marginally significant (see Table 2). As shown in Figure 1, the reductions in SOL discrepancy closely resembled the pattern of the reductions in diary-assessed SOL whereas actigraphy-assessed SOL decreased substantially early in treatment and stayed about the same in the remaining assessment points. In particular, there were elevations in diary-assessed SOL during posttreatment assessments while actigraphy-assessed stayed about the same. Changes in subjective sleep quality showed a similar pattern of change as diary-assessed SOL and SOL discrepancy, but in the opposite direction, indicating improved sleep quality from baseline to follow-up with slight decreases in sleep quality at posttreatment.
Although WASO and TST discrepancies decreased from baseline to posttreatment, the Time by Group interaction did not reach significance (WASO discrepancy: γ=−.41, p=.739; TST discrepancy: γ=5.66, p=.151).
Discussion
The present study examined whether BBTi was efficacious for reducing sleep discrepancy in older adults diagnosed with chronic insomnia in a randomized controlled trial. The main findings were that SOL discrepancy decreased significantly in older adults who received BBTi compared to those who received SMC and that the decreases in SOL discrepancy appeared to be attributable to the changes in diary-assessed SOL but not actigraphy-assessed SOL. Extending from previous findings, this study showed that SOL discrepancy was responsive to behavioral treatment of insomnia without the cognitive component. Nonetheless, we did not find statistically significant Time by Group effects on WASO and TST discrepancies.
Our findings were partially consistent with those in prior studies, in which CBT-I strategies were found effective for reducing SOL discrepancy (Dzierzewski et al., 2019; Kay et al., 2015; Lund et al., 2013). The magnitudes of baseline SOL discrepancy in this sample were similar to those in prior studies and the effect sizes of the changes in sleep discrepancy during the course of the treatment were also comparable. Different from prior studies, BBTi in this study employed only the behavioral component of CBT-I. BBTi in this study also consisted of only four hourly treatment sessions as opposed to eight hourly sessions in Kay et al. and Lund et al. Consistent with our prediction, targeting cognitions related to sleep might not be necessary in addressing sleep discrepancy. Although cognitive arousal contributes to sleep discrepancy (Tang & Harvey, 2004), it appears that sleep restriction, stimulus control, sleep hygiene, and relaxation are adequate for reducing discrepancy in SOL.
The cognitive behavioral model suggests that changing behavior can influence cognitions (Brewin, 1989). BBTi, by inducing behavioral changes, may be sufficient for reducing dysfunctional beliefs about sleep and subsequently decreasing cognitive arousal. Another possible mechanism is that, improved sleep itself, resulting from the behavioral strategies of BBTi, may reduce hyperarousal. As postulated in the hyperarousal model of chronic insomnia, the relationship between insomnia and arousal might be bi-directional. On the one hand, arousal worsens insomnia; on the other, insomnia leads to increased autonomic and cortical activities and altered neuroendocrinal responses, which elevate physiological and cognitive arousal (Riemann et al., 2010). Indeed, the present study converged with prior findings that showed reduction in sleep discrepancy was associated with improvement in sleep quality (Dzierzewski et al., 2019; Lund et al., 2013). Future research on the temporal relationships among dysfunctional thoughts, arousal, insomnia, and sleep discrepancy is needed to disentangle the causal mechanisms underlying these constructs.
We found that the changes of SOL discrepancy appeared to be attributable to changes in diary-assessed SOL rather than changes in actigraphy-assessed SOL. This was not due to actigraphy being insensitive to treatment effects; rather, actigraphy-assessed SOL decreased rapidly early in the treatment and stayed about the same in later assessments (see Figure 1). This indicated that actigraphy was able to detect improvement in SOL. Nonetheless, diary-assessed SOL appeared to vary not entirely as a function of actigraphy-assessed sleep. There were elevations in diary-assessed SOL during posttreatment assessments which were not detected by actigraphy. Given that polysomnography assessments of sleep were not used in the present study, we could not determine if such changes in diary-assessed SOL reflected changes in sleep architecture or other factors.
Although WASO and TST discrepancies decreased from baseline to posttreatment and follow-up, we did not find significantly greater decreases among BBTi participants compared to SMC participants. This finding contradicted findings in Dzierzewski et al. (2019). Dzierzewski et al. conducted t-tests at different assessment points and found significant differences in sleep discrepancy between CBTi and the control group at 3-month, 6-month, and 9-month follow-up assessments while these two groups experienced no significant differences at baseline. In this study, although particiapnts were randomly assigned to either BBTi or SMC, there were significant differences in baseline WASO and TST discrepancies. Hence, the statistical approach used in Dzierzewski et al. (2019) did not suit the data of the present study. Given the significant differences in WASO and TST discrepancies between conditions at baseline, we could not a draw a definitive conclusion about whether BBTi was effective for reducing WASO and TST discrepancies.
The findings regarding WASO and TST discrepancies might likely be sample-specfic. But they could also sugget that the cognitive component in CBTi may have benefit over BBTi in decreasing WASO and TST discrepancies. The cognitive component involves guiding patients to identify their dysfunctionl thoughts about sleep, evaluate the validity of them, examine the consequences of them, and come up with more functional thoughts that do not negatively impact sleep (Michael L. Perlis, Benson-Jungquist, Smith, & Posner, 2005). Although behavioral changes could promote changes in cognitions via alterations of situational cues and feedback (Brewin, 1989), some individuals who are less aware of their dysfunctional thoughts may require more explicit and focused intervention on cognitions to address dysfunctional thoughts. Nevertheless, there is no clear differential theoretical predictions regarding SOL discrepancy versus WASO and TST discrepancies based on the hyperarousal model of insomnia. The mechanisms underlying sleep discrepancy during initital sleep onset and nighttime awakenings pertain to similar altered sensory and information processing during the transitions between sleep and wake states (M. L. Perlis et al., 1997; Riemann et al., 2010). Hence, we speculate that the cognitive component in CBTi may promote quantitatively, rather than qualitatively, greater benefits on reductions in arousal and sleep discrepancy.
The present findings contribute to incremental knowledge regarding the utility of BBTi for improving sleep outcomes in older adults. They also raise questions regarding whether the cognitive component of CBTi would have additional benefits on improving sleep discrepancy, especially WASO and TST discrepancies. Studies on treatment component analysis of CBTi are scarce. An analysis of retrospective reports of the usage of CBTi components during the 1-year follow-up period among adult patients has shown that the usage of sleep restriction and stimulus control were the best predictors of sleep improvements (L. Harvey, Inglis, & Espie, 2002). It is unclear if these findings in adults would generalize to older adults, but there is evidence that shorter interventions may work better for older adults (Irwin, Cole, & Nicassio, 2006). Future comparative trials may help evaluate the differential effectiveness of CBTi and BBTi for sleep discrepancy in older adults controlling for the number of sessions. Future studies that assess arousal directly such as using electroencephalogram (Bastien et al., 2014) may help clarify the role of arousal in the changes of sleep discrepancy during the treatment of insomnia. These studies might be more feasible now as in-home polysomnography and electroencephalogram measures are becoming less costly and more user-friendly in recent years.
The present findings should be interpreted accounting for the following limitations. Actigraphy was used in this study as an objective measure of sleep; however, it should be noted that actigraphy is not the gold-standard objective measure of sleep. Although it has been validated against polysomnography, actigraphy is not a direct measure of sleep stages. Additionally, some studies have shown differential reliabilities and validities regarding actigraphic estimates of different sleep parameters. For instance, the correlations of actigraphy-assessed SOL with polysomnography estimates were medium, compared to strong correlations between actigraphy and polysomnography estimates of WASO and TST (Montserrat Sánchez-Ortuño, Edinger, Means, & Almirall, 2010). The large discrepancy between subjective SOL and actigraphy SOL could be attributable to the inability of actigraphy to detect prolonged motionless wakefulness during sleep onset. This limitation of actigraphy could potentially confound the results of the present study especially the discrepant results regarding SOL as opposed to WASO and TST. The sample of the present study is primarily white. The generalizability of the present findings might be limited to older adults with similar demographics. The sample size of the present study was relatively small. The main study was powered to detect medium effects in the insomnia outcomes. However, because the present study is a secondary analysis, no a priori power analysis was conducted to ensure adequate statistical power for detecting the targeted effects. Furthermore, given the small sample size, BBTi and SMC participants had significant differences in WASO and TST discrepancies at baseline which further limited the interpretation of the non-significant differences at posttreatment and follow-up.
Acknowledgement:
The authors thank Jacob Williams, Dr. Richard B. Berry, and Dr. Michael Marsiske for their consultations on the study design and statistical analysis.
Support: The project described was supported by Award Number AG024459 (Christina S. McCrae, Ph.D, PI) and K23AG049955 (Joseph M. Dzierzewski, PhD, PI) from the National Institute on Aging (NIA). Additional support was provided by an Institutional Training Grant Award Number AG020499 (Michael Marsiske, PhD, Director) from the NIA. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIA.
Footnotes
All authors have approved the manuscript for submission and declare no conflict of interest.
References
- American Psychiatric Association. (2013). Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-5). Diagnostic and Statistical Manual of Mental Disorders 4th Edition TR., 280. 10.1176/appi.books.9780890425596.744053 [DOI] [Google Scholar]
- Bastien CH, Ceklic T, St-Hilaire P, Desmarais F, Pérusse AD, Lefrançois J, & Pedneault-Drolet M (2014). Insomnia and sleep misperception. Pathologie Biologie, 62(5), 241–251. 10.1016/j.patbio.2014.07.003 [DOI] [PubMed] [Google Scholar]
- Brewin CR (1989). Cognitive Change Processes in Psychotherapy. Psychological Review, 96(3), 379–394. 10.1037/0033-295X.96.3.379 [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Germain A, Moul DE, Franzen PL, Brar LK, Fletcher ME, … Monk TH (2011). Efficacy of Brief Behavioral Treatment for Chronic Insomnia in Older Adults. Archives of Internal Medicine, 171(10), 887–895. 10.1001/archinternmed.2010.535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinapoli EA, Gebara MA, Kho T, Butters MA, Gildengers AG, Albert SM, … Karp JF (2017). Subjective-Objective Sleep Discrepancy in Older Adults with MCI and Subsyndromal Depression. Journal of Geriatric Psychiatry and Neurology, 30(6), 316–323. 10.1177/0891988717731827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzierzewski JM, Martin JL, Fung CH, Song Y, Fiorentino L, Jouldjian S, … Alessi CA (2019). CBT for late-life insomnia and the accuracy of sleep and wake perceptions: Results from a randomized-controlled trial. Journal of Sleep Research, (September 2018), e12809. 10.1111/jsr.12809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edinger JD, Bonnet MH, Bootzin RR, Doghramji K, Dorsey CM, Espie CA, … American Academy of Sleep Medicine Work Group. (2006). Derivation of research diagnostic criteria for insomnia: report of an American Academy of Sleep Medicine Work Group. Sleep, 27(8), 1567–1596. Retrieved from http://www.ncbi.nlm.nih.gov/sites/entrez?Db=pubmed&DbFrom=pubmed&Cmd=Link&LinkName=pubmed_pubmed&LinkReadableName=RelatedArticles&IdsFromResult=15683149&ordinalpos=3&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum [DOI] [PubMed] [Google Scholar]
- Harvey AG, & Tang NKY (2012). (Mis)perception of sleep in insomnia: A puzzle and a resolution. Psychological Bulletin, 138(1), 77–101. 10.1037/a0025730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey L, Inglis SJ, & Espie CA (2002). Insomniacs’ reported use of CBT components and relationship to long-term clinical outcome. Behaviour Research and Therapy, 40(1), 75–83. 10.1016/S0005-7967(01)00004-3 [DOI] [PubMed] [Google Scholar]
- Hsiao F-C, Tsai P-J, Wu CW, Yang C-M, Lane TJ, Lee H-C, … Wu Y-Z (2018). The neurophysiological basis of the discrepancy between objective and subjective sleep during the sleep onset period: an EEG-fMRI study. Sleep, 41(6), zsy056–zsy056. 10.1093/sleep/zsy056 [DOI] [PubMed] [Google Scholar]
- Irwin MR, Cole JC, & Nicassio PM (2006). Comparative meta-analysis of behavioral interventions for insomnia and their efficacy in middle-aged adults and in older adults 55+ years of age. Health Psychology, 25(2), 3–14. 10.1037/0278-6133.25.1.3 [DOI] [PubMed] [Google Scholar]
- Kay DB, Buysse DJ, Germain A, Hall M, & Monk TH (2015). Subjective-objective sleep discrepancy among older adults: Associations with insomnia diagnosis and insomnia treatment. Journal of Sleep Research, 24(1), 32–39. 10.1111/jsr.12220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koffel E, Bramoweth AD, & Ulmer CS (2018). Increasing access to and utilization of cognitive behavioral therapy for insomnia (CBT-I): a narrative review. Journal of General Internal Medicine, 33(6), 955–962. 10.1007/s11606-018-4390-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichstein KL, & Morin CM (Eds.). (2000). Treatment of Late-Life Insomnia. Sage Publications. [Google Scholar]
- Lund HG, Rybarczyk BD, Perrin PB, Leszczyszyn D, & Stepanski E (2013). The discrepancy between subjective and objective measures of sleep in older adults receiving cbt for comorbid insomnia. Journal of Clinical Psychology, 69(10), 1108–1120. 10.1002/jclp.21938 [DOI] [PubMed] [Google Scholar]
- McCrae C, Curtis AF, Williams JM, Dautovich ND, McNamara JPH, Stripling A, … Marsiske M (2018). Efficacy of brief behavioral treatment for insomnia in older adults: examination of sleep, mood, and cognitive outcomes. Sleep Medicine, 51, 153–166. 10.1016/j.sleep.2018.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrae C, Rowe MA, Tierney CG, Dautovich ND, DeFinis AL, & McNamara JPH (2005). Sleep Complaints, Subjective and Objective Sleep Patterns, Health, Psychological Adjustment, and Daytime Functioning in Community-Dwelling Older Adults. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences, 60(4), P182–P189. 10.1093/geronb/60.4.p182 [DOI] [PubMed] [Google Scholar]
- Montserrat Sánchez-Ortuño M, Edinger JD, Means MK, & Almirall D (2010). Home is where sleep is: An ecological approach to test the validity of actigraphy for the assessment of insomnia. Journal of Clinical Sleep Medicine, 15(6), 21–29. [PMC free article] [PubMed] [Google Scholar]
- Morin CM, Colecchi C, Stone J, Sood R, & Brink D (1999). Behavioral and pharmacological therapies for late-life insomnia: A randomized controlled trial. Journal of the American Medical Association, 281(11), 991–999. 10.1001/jama.281.11.991 [DOI] [PubMed] [Google Scholar]
- O’Donnell D, Silva EJ, MÜnch M, Ronda JM, Wang W, & Duffy JF (2009). Comparison of subjective and objective assessments of sleep in healthy older subjects without sleep complaints. Journal of Sleep Research, 18(2), 254–263. 10.1111/j.1365-2869.2008.00719.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohayon MM, Carskadon MA, Guilleminault C, & Vitiello MV (2003). Ohayon et al. - 2003 - Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals Developing normat. 31(2), 20–23. [DOI] [PubMed] [Google Scholar]
- Perlis ML, Giles DE, Mendelson WB, Bootzin RR, & Wyatt JK (1997). Psychophysiological insomnia: The behavioural model and a neurocognitive perspective. Journal of Sleep Research, 6(3), 179–188. 10.1046/j.1365-2869.1997.00045.x [DOI] [PubMed] [Google Scholar]
- Perlis Michael L., Benson-Jungquist C, Smith MT, & Posner DA (2005). Cognitive behavioral treatment of insomnia: A session-by-session guide. Springer. [Google Scholar]
- Qaseem A, Kansagara D, Forciea MA, Cooke M, Denberg TD, & Clinical Guidelines Committee of the American College of, P. (2016). Management of Chronic Insomnia Disorder in Adults: A Clinical Practice Guideline From the American College of Physicians. Ann Intern Med, 165(2), 125–133. 10.7326/M15-2175 [DOI] [PubMed] [Google Scholar]
- Rezaie L, Fobian AD, McCall WV, & Khazaie H (2018). Paradoxical insomnia and subjective–objective sleep discrepancy: A review. Sleep Medicine Reviews, Vol. 40, pp. 196–202. 10.1016/j.smrv.2018.01.002 [DOI] [PubMed] [Google Scholar]
- Riemann D, Spiegelhalder K, Feige B, Voderholzer U, Berger M, Perlis M, & Nissen C (2010). The hyperarousal model of insomnia: A review of the concept and its evidence. Sleep Medicine Reviews, 14(1), 19–31. 10.1016/j.smrv.2009.04.002 [DOI] [PubMed] [Google Scholar]
- Tang NK, & Harvey AG (2004). Effects of cognitive arousal and physiological arousal on sleep perception. Sleep, 27(1), 69–78. 10.1093/sleep/27.1.69 [DOI] [PubMed] [Google Scholar]
- Tsuchiyama K, Nagayama H, Kudo K, Kojima K, & Yamada K (2003). Discrepancy between subjective and objective sleep in patients with depression. Psychiatry and Clinical Neurosciences, 57(3), 259–264. 10.1046/j.1440-1819.2003.01114.x [DOI] [PubMed] [Google Scholar]
- Weiss AR, Johnson NL, Berger NA, & Redline S (2010). Validity of activity-based devices to estimate sleep. Journal of Clinical Sleep Medicine. [PMC free article] [PubMed] [Google Scholar]
- Williams JM, Kay DB, Rowe M, & McCrae CS (2013). Sleep discrepancy, sleep complaint, and poor sleep among older adults. Journals of Gerontology - Series B Psychological Sciences and Social Sciences, 68(5), 712–720. 10.1093/geronb/gbt030 [DOI] [PMC free article] [PubMed] [Google Scholar]