Abstract
In dermatopathological daily practice, vertical histopathology sections are classically used to analyze skin biopsies. Conversely, horizontal histopathological sections are currently used for the diagnosis of some types of alopecia. In the last years the morphological findings obtained by horizontal histopathology have been correlated to those obtained by in vivo reflectance confocal microscopy which provides the same “point of view” of the skin. This review paper emphasizes the strong matching and correlation between reflectance confocal microscopy images and horizontal histopathology in cutaneous neoplasms, further demonstrating the strong reliability of this innovative, non-invasive technique in the management of skin tumors.
Keywords: horizontal histopathology, reflectance confocal microscopy, skin cancer, correlation, horizontal histopathological sections
Introduction
One of the major application fields of dermatological research has always been the identification of new diagnostic tools capable of improving the diagnostic precocity and accuracy of skin neoplasms (1, 2). In the last decade, in vivo reflectance confocal microscopy (RCM) is gradually establishing itself as a non-invasive diagnostic technique for several skin diseases, being able to provide a horizontal high-resolution “point of view” of the skin, from the stratum corneum to the papillary dermis; horizontal skin images up to a 250 μm of maximum depth may be studied through this technique (3–6). The use of RCM in the diagnostic approach to many inflammatory and neoplastic skin diseases is still increasing, representing one of the major diagnostic aids in the dermatological clinical practice (7). However, the horizontal “point of view” provided by RCM does not allow an optimal correlation with classical histopathology that, as known, produces a full-thickness vertical overview of the skin (8, 9). Instead, horizontal histological sections (HHSs) allow a better correlation as they reflect the same skin plane observed by RCM (10).
The possibility of optimally comparing horizontal histopathology and RCM images represents a relatively new trend, and quite a few papers have been published in this field regarding both inflammatory and neoplastic disorders (11–17). The purpose of this review paper is to establish the “state of the art” on RCM and HHS findings in skin tumors, emphasizing how well horizontal histopathology reflects the images provided by RCM.
Squamous Cell Carcinoma in Situ (Bowen’s Disease)
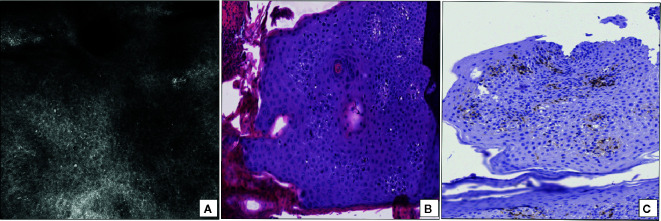
Squamous cell carcinoma in situ (SCCis) represents the earliest and non-invasive form of squamous cell carcinoma, in which, by definition, the neoplastic cells do not infiltrate the basement membrane and therefore lack distant metastatic potential (14). SCCis mainly affects photoexposed skin of elderly, and the head and neck are the most commonly affected sites (14). Clinically, SCCis arises in the form of flat/raised, reddish/brownish in color, often scaly, papules or plaques; due to the low specificity of the clinical presentation, further non-invasive diagnostic tools, such as dermoscopy and RCM, are often required to enhance the diagnostic accuracy of SCCis (14, 18). The detection of “red dots”, representing glomerular vessels in the superficial dermis, is the most typical dermoscopic finding of SCCis (18). In addition, RCM has been also validated as useful diagnostic tool and its application in the dermatological practice has been supported by the perfect matching with HHS found by our research group (14). SCCis shows the following RCM features (14) ( Figures 1A, C ): i) at the level of stratum corneum, highly refractive amorphous structures and sporadically polygonal, nucleated cells; ii) at the level of the stratum granulosum/spinosum, marked architectural disarray, consisting of keratinocytes highly variable in size, shape, and nuclear morphology; scattered bright dendritic cells may also be found; iii) at the level of the dermoepidermal junction, large rounded dark areas, corresponding to enlarged dermal papillae. Horizontal histopathology perfectly matches with the previous reported RCM findings (14) ( Figures 1B, D ): hyperkeratosis and parakeratosis are the histopathological causes of the refractive amorphous structures and the nucleated cells observed in the stratum corneum at RCM; the loss of architectural array visible in the stratum granulosum/spinosum at RCM reflects the presence of atypical keratinocytes with nuclei of variable size and shape along the entire thickness of epidermis; some S-100 positive, CD1a negative and Melan-A negative dendritic cells may be occasionally found scattered among the neoplastic cells; lastly, at the dermoepidermal junction, HHSs show enlarged dermal papillae containing glomeruloid capillary vessels, corresponding both to the rounded dark areas and to the “red dots” observed at RCM and dermoscopy, respectively. Since the horizontal histopathology does not allow to evaluate the possible presence of dermal invasion, the concept that its use is only for the purpose of comparing it with the RCM findings, in order to further validate the diagnostic use of RCM, must be emphasized.
Figure 1.
Squamous cell carcinoma in situ. (A) RCM image at the stratum spinosum showing a marked loss of the normal honeycomb pattern (architectural disarray) due to the presence of markedly variable size, shape, and nuclear morphology keratinocytes. (B) Horizontal histopathology at the same level revealing neoplastic keratinocytes with high-grade nuclear atypia (hematoxylin and eosin; original magnification 400×). (C) RCM image at the dermoepidermal junction showing dilated blood vessels within enlarged edged dermal papillae. (D) Horizontal histopathology at the same level confirming the RCM finding (hematoxylin and eosin; original magnification 100×).
Mycosis Fungoides With Patch Lesions
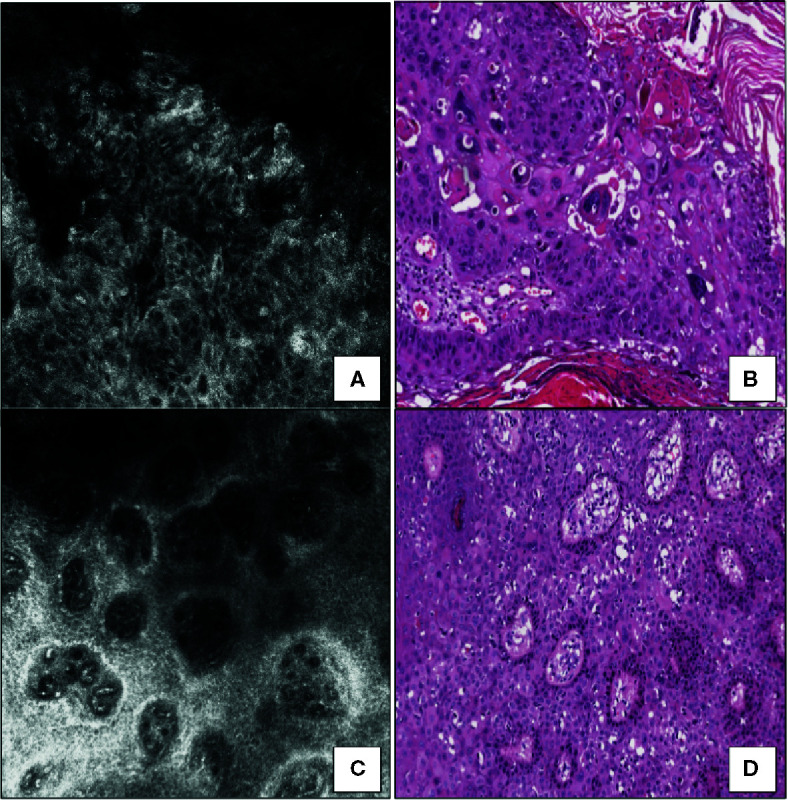
Mycosis fungoides (MF) is the most frequent T-cell lymphoma of the skin and seems pathogenetically related to a monoclonal T-cell receptor (TCR) gene rearrangement, leading to a monoclonal proliferation of cutaneous CD4-positive T lymphocytes (19, 20). Clinically, MF exhibits a higher predilection for dark skin (2:1) males (2:1) and, in its classical form, presents a slow-growing clinical course with a progressive shift from patches to plaques and, in final stages, tumors (19, 20). A variable combination of patches, plaques and tumors is frequently observed in MF with tumor lesions (20). Both clinical presentation and histopathology of MF are often non-specific, especially when it occurs in the form of patchy lesions, to such an extent that multiple biopsies are often necessary to obtain a definitive diagnosis (19, 21). RCM may improve the diagnostic accuracy of MF (13, 22, 23). In the upper portion of epidermis, epidermal disarray with disruption of the normal “honeycomb” appearance and sometimes hyporefractive areas, combined to the detection of small sized bright cells interspersed within epidermal layers are usually identifiable with RCM (13) ( Figure 2A ); the same bright cells are found at the dermoepidermal junction both inside and around dermal papillae, visible as round darker areas (13). RCM features of MF perfectly match with HHS (13): the presence of spongiosis, epidermotropic CD4-positive lymphocytes ( Figures 2B, C ) forming Pautrier’s microabscesses and band-like distributed CD4-positive lymphocytes at dermoepidermal junction are the histopathological “mirror” of what is detectable with RCM. In addition, the differential diagnosis with eczematous disorders can become more straightforward using RCM (13), that shows in the stratum spinosum widespread round, deeply dark areas, intercellular spaces and few mildly bright cells: these findings are confirmed by horizontal histopathology, displaying marked spongiotic features combined to a less conspicuous lymphocytic exocytosis than MF (13).
Figure 2.
Mycosis fungoides with patch lesions. (A) RCM at the stratum spinosum revealing a diffuse epidermal disarray with scattered small hyperreflective cells (epidermotropic lymphocytes). (B) Horizontal histopathology at the same level showing the presence of lymphocyte epidermotropism (hematoxylin and eosin; original magnification 400×). (C) Immunohistochemical staining for CD4 revealing the CD4-positive phenotype of epidermotropic T-lymphocytes (immunoperoxidase staining; original magnification 350×).
Eccrine Poroma
Eccrine poroma (EP) is a sweat gland derived adnexal tumor, first described by Pinkus in 1956 (24), that clinically arises as a slow-growing, sometimes ulcerated, reddish, and firm in consistency nodule, mostly located to the acral regions (25, 26). Usually, EP has a benign clinical course, even if a malignant counterpart, called “porocarcinoma” and characterized by low distant metastatic potential, has been also described (27). EP usually occurs on photodamaged skin, mimicking cutaneous malignancies, such as basal cell carcinoma (BCC), squamous cell carcinoma (SCC) or malignant melanoma (MM) (25, 26). Although the definitive diagnosis of EP is still based on conventional histopathology, non-invasive techniques, including dermoscopy and RCM, allow ruling out malignant conditions, and to suspect a benign adnexal neoplasm (28, 29). Dermoscopically, EP usually presents milky red areas at the periphery of the lesion and a polymorphous vascular pattern in the center, including glomerular, flower-like and dotted vessels (30). RCM shows a uniformly well-circumscribed neoplasm, consisting of hyper-reflective clusters surrounded by a darker stromal component (28, 30). Neoplastic cells are bright and homogeneous in size and shape, with round and dark nuclei, and may be arranged around non-reflective rounded areas (28, 30). Deeper sections show a richly vascularized stroma intermingled with tumor nests (28, 30). RCM images of EP correspond well with HHS (28, 30): neoplastic cells are monomorphic, cuboid-shaped, arranged in basaloid nests and occasionally forming round/slit-like ducts with eosinophilic material inside; these ducts strongly match with the non-reflective round dark areas visible with RCM and represent foci of ductal differentiation of EP. Bright uniformly shaped and sized cells interspersed within the tumor island or scattered in the upper dermis are often present at RCM in the pigmented variant of EP (28); these cells histologically correspond to melanocytes and melanophages, respectively. The presence of melanocytes in pigmented EP makes the differential diagnosis with MM mandatory: neoplastic melanocytes in MM are usually more irregularly shaped/denditric or fusiform than those observed in pigmented EP (31, 32).
Disseminated Superficial Actinic Porokeratosis
Disseminated superficial actinic porokeratosis (DSAP) represents the most frequent variant of porokeratosis. It clinically presents as multiple scaly macules with a whitish central area surrounded by a slightly raised rim that mainly occurs on photoexposed regions (33). Dermoscopy frequently shows a double free edged scaly rim, whitish in color, representing the dermoscopic equivalent of the cornoid lamella, that is the histopathological hallmark of porokeratosis (34, 35). RCM may be useful in the diagnostic approach to DSAP, and its finding has been validated on the basis of the correlation with HHS (36). At RCM, architectural disarray with loss of the normal “honeycomb” pattern is observed in the center of the lesion (36); proceeding towards the periphery, a less refractile destructured area, containing more refractile amorphous substance (cornoid lamella) and surrounded by normal skin with regular “honeycomb” array, is found (36). HHS strongly matches with these RCM features and shows columns of parakeratosis (cornoid lamella) combined with moderately atypical keratinocytes (36).
Solitary Mastocytoma
The term “mastocytosis” includes a wide spectrum of diseases caused by a clonal proliferation of mast cell and affecting simultaneously or at different times several organs, including the skin, bone marrow, liver, spleen, and lymphatic system (37). Based on the involved organs, the World Health Organization identifies two different variants of mastocytosis: cutaneous mastocytosis, if the disease exclusively affects the skin, and systemic mastocytosis, if there are other organs affected, regardless of the skin. Furthermore, cutaneous mastocytosis may be clinically further subdivided into maculo-papular cutaneous mastocytosis, diffuse cutaneous mastocytosis, and cutaneous mastocytoma (38). The latter includes not only the cases when there is a single cutaneous lesion (solitary mastocytoma; SM), but also those in which up to three skin lesions are seen (38). Clinical presentation of SM is variable and ranges from brownish/reddish macules to papules, plaques and nodules, showing swelling spontaneously or after rubbing (Darier’s sign). Zhang et al. (39) first described RCM findings of mastocytosis in a huge group of 200 patients, including all different clinical presentation; regardless of the specific variant examined; all cases showed similar RCM features: the absence of aggregates of bright element in the context of finely granular and edematous papillary dermis was a constant finding. Following these results, our group first described more specific RCM features of SM and correlated them with HSS for validation (15): in particular, the presence of enlarged dermal papillae, containing tortuous vessels and large, uniformly round-shaped, bright cells at the level of dermoepidermal junction ( Figure 3A ) perfectly matched with the finding of aggregates of round, CD117-positive mastocytes with granular cytoplasm located to dermal papillae on HHS ( Figure 3B ).
Figure 3.
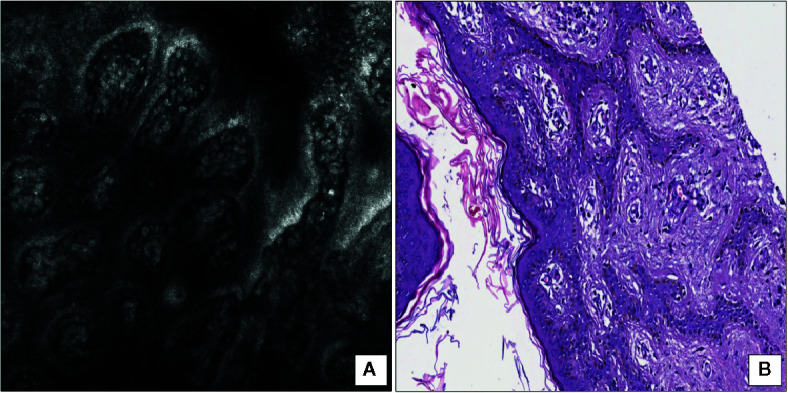
Solitary mastocytoma. (A) RCM at the level of dermoepidermal junction showing multiple, large and rounded bright cells within dilated dermal papillae. (B) Horizontal histopathology at the same level revealing the presence of round mastocytes with pale and granular cytoplasm within dermal papillae (hematoxylin and eosin; original magnification 150×).
Melanocytic Tumors
While the introduction of dermoscopy has definitely represented a turning point in the diagnostic accuracy of melanocytic tumors, allowing the detection of some architectural patterns corresponding to specific histopathological features, in recent years RCM has emerged as a valid tool capable of providing architectural and morphological information at the cellular level (40–42); in particular, the combined use of dermoscopy and RCM proved to increase the accuracy for facial tumor detection, compared with RCM alone (43).
Braga et al. (17) compared RCM findings of melanocytic tumors and HHS. They selected four MMs and two benign nevi and compared specific dermoscopic patterns of cutaneous MM such as pigment network, irregular globules and pseudopods, and their benign counterparts, detectable in nevi, to RCM findings and both vertical and horizontal histopathology. Regarding the pigment network, two melanomas showed two different types of atypical network: the first MM presented on RCM a proliferation of bright dendritic cells at the level of dermoepidermal junction, some of them protruding from the epidermis to the superficial dermis to form “bridges”; conventional vertical histopathology revealed an in situ melanoma, and HHS showed the same features observed on RCM, confirming the presence of many atypical Melan-A-positive melanocytes surrounding dermal papillae and bulging into dermis. RCM of the second MM with an atypical pigmented network showed at dermoepidermal junction atypical nests of both rounded and elongated hyperreflective melanocytes combined to an architectural disarray of dermal papillae and some bright cells or small dots within dermal papillae; vertical histopathology revealed an in situ melanoma, and RCM findings were confirmed by HHS showing pleomorphic melanocytes arranged in nests and presence of lymphocytes within dermal papillae. Based of RCM, Braga et al. (17) were also able to discriminate dermoscopic globules in nevi and melanomas on the basis of morphological atypia: both RCM and HHS showed small nests of monomorphous non-atypical bright melanocytes non-connected with epithelium in nevi and larger nests of pleomorphic neoplastic melanocytes in MMs. Lastly, pseudopods were not characterized by morphological atypia on RCM, corresponding to peripherally visible, confluent clusters of pigmented neoplastic melanocytes on horizontal histopathology. Navarrete-Dechent et al. (16) also matched the dermoscopic sign “circle within a circle” of lentigo maligna (presence of pigmentation within and around hair follicles) with its RCM and HHS: RCM revealed the presence of hair follicles surrounded by numerous dendritic bright melanocytes and layers of keratinocytes filled at the periphery with rounded/elongated hyperreflective melanocytes. HHS strongly overlapped with RCM, showing a high pigmentation of the keratinocytes of the basal layer of the epidermis combined with an increased number of junctional melanocytes.
As previously mentioned regarding SCCis, also for melanocytic tumors, the use of horizontal histopathology has only the purpose of validating the RCM application in clinical practice without replacing conventional histopathology as diagnostic gold standard.
Discussion
In dermatology, the majority of skin specimens from biopsy or surgical procedures is analyzed using classical vertical histopathological sections, which represents the diagnostic gold standard. Horizontal histopathology is currently used for the diagnosis of some types of alopecia allowing a more correct visualization of follicular and perifollicular features (44).
More recently, HHS has been used to correlate with the morphological features obtained by RCM which provides the same transversal “point of view” of the skin. In particular, the strong matching and correlation between RCM images and HHS in skin tumors ( Table 1 ), as shown in this review, further demonstrates the reliability of this innovative, non-invasive technique in the management of skin tumors. Based on such correlations, some considerations can be made: in SCCis and melanoma RCM may confirm the clinical suspect addressing the correct therapeutic approach; in clinically atypical SM, RCM evaluation may avoid biopsy or excision as it is generally self-resolving; in MF and DSAP, RCM is particularly useful for the selection of the best site for biopsy thus avoiding multiple biopsies often quite bothersome for the patient; a further application of RCM in skin tumors may consist in the early recognition of local recurrences after medical or surgical treatments of the disease (14).
Table 1.
Correlation between reflectance confocal microscopy and horizontal histopathology in skin tumors: summary.
| Depth | RCM | HHS | |
|---|---|---|---|
| SCCis (14) | Stratum Corneum | - Hyperrefractive amorphous structures - Polygonal, nucleated cells |
- Hyperkeratosis - Parakeratosis |
| Stratum granulosum/spinosum | - Architectural disarray - Bright dendritic cells |
- Large atypical keratinocytes - Langerhans cells (S-100 +, CD1a+, Melan-A -) |
|
| Dermoepidermal junction | - Enlarged edged papillae with widened dermal papillae - Tortuouscapillary vessels |
- Enlarged papillae with widened dermal papillae - Tortuouscapillary vessels |
|
| MF (13) | Upper epidermis | - Darker spots compared to the surrounding epidermis. - Epidermal disarray and presence of small bright cells |
- Spongiosis - CD4-positive T-cellepidermotropism |
| Dermoepidermal junction | - Small bright cells scattered within and among roundish hyporefractive areas (dermal papillae) | - CD4-positive lymphocytes infiltrating dermal papillae | |
| EP (28, 30) | Epidermis | - Clusters of small, hyperrefractive and uniformly shaped cells with round dark nuclei surrounded by keratin - Parakeratosis |
- Monomorphic basophilic neoplastic cells with large and round nuclei surrounded by amorphic keratin - Parakeratosis |
| Dermis | - Larger and confluent cell clusters embedded in a denser and highly vascularized stroma- Neoplastic clusters arranged around darker hyporefractive rounded areas- Presence of bright, uniformly shaped and sized cells interspersed within tumor island or scattered in the upper dermis (pigmented variant) | - Increased tumor volume and denser and more vascularized stromal compartment - Intratumoral round or slit-like areas filled with eosinophilic substance (spots of ductal differentiation) - Intratumoral melanocytes or melanophages (pigmented variant) |
|
| DSAP (36) | Epidermis | - Architectural disarray with loss of the normal “honeycomb” pattern (central zone) - Hyperrefractive amorphous material (cornoid lamella) within hyporefractivedestructured areas, surrounded by skin with regular “honeycomb” pattern (peripheral zone) |
- Columns of parakeratosis (cornoid lamella) combined with moderately atypical keratinocytes |
| SM (15) | Dermoepidermal junction | - Tortuous vessels and large, uniformly round-shaped, bright cells within enlarged dermal papillae | - Dermal papillae containing aggregates of round, CD117-positive mastocytes with granular cytoplasm |
| MTs (16, 17) | Dermoepidermal junction | - Atypical pigment network: proliferation of bright dendritic cells, forming “bridge” from epidermis to the superficial dermis (in situ melanoma) - Atypical pigment network: atypical nests of rounded and spindled hyperreflective melanocytes combined to an architectural disarray of dermal papillae and some bright cells or small dots within dermal papillae (in situ melanoma) - Hair follicles surrounded by multiple dendritic bright melanocytes and layers of keratinocytes filled at the periphery with rounded/elongated hyperreflective melanocytes (lentigo maligna). |
- Presence of atypical Melan-A-positive melanocytes surrounding dermal papillae and bulging into dermis (in situ melanoma) - Atypical melanocytes arranged in nests and presence of lymphocytes within dermal papillae (in situ melanoma) - Heavily pigmented keratinocytes of the basal layer of the epidermis combined with an increased number of junctional melanocytes (lentigo maligna). |
| Upperdermis | - Dermoscopic globules: small nests of monomorphous non-atypical bright melanocytes non connected with epithelium in nevi and larger nests of pleomorphic neoplastic melanocytes in melanomas - Non-atypical peripheral pseudopods |
- Small nests of non-atypical melanocytes in nevi and larger clusters of atypical neoplastic melanocytes in melanomas - peripheral confluent clusters of pigmented neoplastic melanocytes |
RCM, reflectance confocal microscopy; HHS, horizontal histopathological section; SCCis, squamous cell carcinoma in situ; MF, mycosis fungoides; EP, eccrine poroma; DSAP, disseminated superficial actinic porokeratosis; SM, solitary mastocytoma; MTs, melanocytic tumours.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- 1. Pehamberger H, Steiner A, Wolff K. In vivo epiluminescence microscopy of pigmented skin lesions. I. Pattern analysis of pigmented skin lesions. J Am Acad Dermatol (1987) 17:571–83. 10.1016/s0190-9622(87)70239-4 [DOI] [PubMed] [Google Scholar]
- 2. Argenziano G, Soyer HP, Chimenti S, Talamini R, Corona R, Sera F, et al. Dermoscopy of pigmented skin lesions: results of a consensus meeting via the Internet. J Am Acad Dermatol (2003) 48:679–93. 10.1067/mjd.2003.281 [DOI] [PubMed] [Google Scholar]
- 3. Ardigo M, Agozzino M, Franceschini C, Lacarrubba F. Reflectance Confocal Microscopy Algorithms for Inflammatory and Hair Diseases. Dermatol Clin (2016) 34:487–96. 10.1016/j.det.2016.05.011 [DOI] [PubMed] [Google Scholar]
- 4. Lacarrubba F, Verzì AE, Pippione M, Micali G. Reflectance confocal microscopy in the diagnosis of vesicobullous disorders: case series with pathologic and cytologic correlation and literature review. Skin Res Technol (2016) 22:479–86. 10.1111/srt.12289 [DOI] [PubMed] [Google Scholar]
- 5. Lacarrubba F, Boscaglia S, Nasca MR, Caltabiano R, Micali G. Grover’s disease: dermoscopy, reflectance confocal microscopy and histopathological correlation. Dermatol Pract Concept (2017) 7:51–4. 10.5826/dpc.0703a11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lacarrubba F, Verzì AE, Ardigò M, Micali G. Handheld reflectance confocal microscopy for the diagnosis of molluscum contagiosum: histopathology and dermoscopy correlation. Australas J Dermatol (2017) 58:e123–5. 10.1111/ajd.12511 [DOI] [PubMed] [Google Scholar]
- 7. Guitera P, Menzies SW, Longo C, Cesinaro AM, Scolyer RA, Pellacani G. In vivo confocal microscopy for diagnosis of melanoma and basal cell carcinoma using a two-step method: analysis of 710 consecutive clinically equivocal cases. J Invest Dermatol (2012) 132:2386–94. 10.1038/jid.2012.172 [DOI] [PubMed] [Google Scholar]
- 8. Pellacani G, Longo C, Malvehy J, Puig S, Carrera C, Segura S, et al. In vivo confocal microscopic and histopathologic correlations of dermoscopic features in 202 melanocytic lesions. Arch Dermatol (2008) 144:1597–608. 10.1001/archderm.144.12.1597 [DOI] [PubMed] [Google Scholar]
- 9. Soyer HP, Kenet RO, Wolf IH, Kenet BJ, Cerroni L. Clinicopathological correlation of pigmented skin lesions using dermoscopy. Eur J Dermatol (2000) 10:22–8. [PubMed] [Google Scholar]
- 10. Rezze GG, Scramim AP, Neves RI, Landman G. Structural correlations between dermoscopic features of cutaneous melanomas and histopathology using transverse sections. Am J Dermatopathol (2006) 28:13–20. 10.1097/01.dad.0000181545.89077.8c [DOI] [PubMed] [Google Scholar]
- 11. Verzì AE, Lacarrubba F, Caltabiano R, Broggi G, Musumeci ML, Micali G. Reflectance Confocal Microscopy Features of Plaque Psoriasis Overlap With Horizontal Histopathological Sections: A Case Series. Am J Dermatopathol (2019) 41:355–7. 10.1097/DAD.0000000000001297 [DOI] [PubMed] [Google Scholar]
- 12. Lacarrubba F, Verzì AE, Caltabiano R, Broggi G, Di Natale A, Micali G. Discoid lupus erythematosus: Reflectance confocal microscopy features correlate with horizontal histopathological sections. Skin Res Technol (2019) 25:242–4. 10.1111/srt.12636 [DOI] [PubMed] [Google Scholar]
- 13. Broggi G, Lacarrubba F, Verzì AE, Micali G, Caltabiano R. Confocal microscopy features of patch-stage mycosis fungoides and their correlation with horizontal histopathological sections. A Case Series. J Cutan Pathol (2019) 46:163–5. 10.1111/cup.13384 [DOI] [PubMed] [Google Scholar]
- 14. Broggi G, Verzì AE, Lacarrubba F, Caltabiano R, Di Natale A, Micali G. Correlation between reflectance confocal microscopy features and horizontal histopathology in cutaneous squamous cell carcinoma in situ: A case series. J Cutan Pathol (2020) 47:777–80. 10.1111/cup.13708 [DOI] [PubMed] [Google Scholar]
- 15. Verzì AE, Lacarrubba F, Caltabiano R, Dinotta F, Micali G. Reflectance confocal microscopy of solitary mastocytoma and correlation with horizontal histopathological sections. Skin Res Technol (2020). 10.1111/srt.12902 [DOI] [PubMed] [Google Scholar]
- 16. Navarrete-Dechent C, Liopyris K, Cordova M, Busam KJ, Marghoob AA, Chen CJ. Reflectance Confocal Microscopic and En Face Histopathologic Correlation of the Dermoscopic “Circle Within a Circle” in Lentigo Maligna. JAMA Dermatol (2018) 154:1092–4. 10.1001/jamadermatol.2018.2216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Braga JC, Macedo MP, Pinto C, Duprat J, Begnami MD, Pellacani G, et al. Learning reflectance confocal microscopy of melanocytic skin lesions through histopathologic transversal sections. PLoS One (2013) 8:e81205. 10.1371/journal.pone.0081205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ianoși SL, Batani A, Ilie MA, Tampa M, Georgescu SR, Zurac S, et al. Non-invasive imaging techniques for the in vivo diagnosis of Bowen’s disease: Three case reports. Oncol Lett (2019) 17:4094–101. 10.3892/ol.2019.10079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ahn CS, ALSayyah A, Sangüeza OP. Mycosis fungoides: an updated review of clinicopathologic variants. Am J Dermatopathol (2014) 36:933–48; quiz 949-51. 10.1097/DAD.0000000000000207 [DOI] [PubMed] [Google Scholar]
- 20. Cerroni L. Mycosis fungoides-clinical and histopathologic features, differential diagnosis, and treatment. Semin Cutan Med Surg (2018) 37:2–10. 10.12788/j.sder.2018.002 [DOI] [PubMed] [Google Scholar]
- 21. Mancebo SE, Cordova M, Myskowski PL, Flores ES, Busam K, Jawed SI, et al. Reflectance confocal microscopy features of mycosis fungoides and Sézary syndrome: correlation with histopathologic and T-cell receptor rearrangement studies. J Cutan Pathol (2016) 43:505–15. 10.1111/cup.12708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li W, Dai H, Li Z, Xu AE. Reflectance confocal microscopy for the characterization of mycosis fungoides and correlation with histology: a pilot study. Skin Res Technol (2013) 19:352–5. 10.1111/srt.12049 [DOI] [PubMed] [Google Scholar]
- 23. Lacarrubba F, Ardigò M, Di Stefani A, Verzì AE, Micali G. Dermatoscopy and Reflectance Confocal Microscopy Correlations in Nonmelanocytic Disorders. Dermatol Clin (2018) 36:487–501. 10.1016/j.det.2018.05.015 [DOI] [PubMed] [Google Scholar]
- 24. Goldman P, Pinkus H, Rogin JR. Eccrine poroma; tumours exhibiting features of the epidermal sweat duct unit. AMA Arch Derm (1956) 74:511–21. 10.1001/archderm.1956.01550110055013 [DOI] [PubMed] [Google Scholar]
- 25. Chessa MA, Patrizi A, Baraldi C, Fanti PA, Barisani A, Vaccari S. Dermoscopic-Histopathological Correlation of Eccrine Poroma: An Observational Study. Dermatol Pract Concept (2019) 9:283–91. 10.5826/dpc.0904a07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ferrari A, Buccini P, Silipo V, De Simone P, Mariani G, Marenda S, et al. Eccrine poroma: a clinical-dermoscopic study of seven cases. Acta Derm Venereol (2009) 89:160–4. 10.2340/00015555-0608 [DOI] [PubMed] [Google Scholar]
- 27. Parra O, Kerr DA, Bridge JA, Loehrer AP, Linos K. A case of YAP1 and NUTM1 rearranged porocarcinoma with corresponding immunohistochemical expression: Review of recent advances in poroma and porocarcinoma pathogenesis with potential diagnostic utility. J Cutan Pathol (2020) 48:95–101. 10.1111/cup.13832 [DOI] [PubMed] [Google Scholar]
- 28. Tachihara R, Choi C, Langley RG, Anderson RR, González S. In vivo confocal imaging of pigmented eccrine poroma. Dermatology (2002) 204:185–9. 10.1159/000057879 [DOI] [PubMed] [Google Scholar]
- 29. Moscarella E, Zalaudek I, Agozzino M, Vega H, Cota C, Catricalà C, et al. Reflectance confocal microscopy for the evaluation of solitary red nodules. Dermatology (2012) 224:295–300. 10.1159/000339339 [DOI] [PubMed] [Google Scholar]
- 30. Schirra A, Kogut M, Hadaschik E, Enk AH, Haenssle HA. Eccrine poroma: correlation of reflectance confocal microscopy and histopathology of horizontal sections. J Eur Acad Dermatol Venereol (2016) 30:e167–9. 10.1111/jdv.13473 [DOI] [PubMed] [Google Scholar]
- 31. Langley RG, Rajadhyaksha M, Dwyer PJ, Sober AJ, Flotte TJ, Anderson RR. Confocal scanning laser microscopy of benign and malignant melanocytic skin lesions in vivo . J Am Acad Dermatol (2001) 45:365–76. 10.1067/mjd.2001.117395 [DOI] [PubMed] [Google Scholar]
- 32. Busam KJ, Hester K, Charles C, Sachs DL, Antonescu CR, Gonzalez S, et al. Detection of clinically amelanotic malignant melanoma and assessment of its margins by in vivo confocal scanning laser microscopy. Arch Dermatol (2001) 137:923–9. [PubMed] [Google Scholar]
- 33. Gu CY, Zhang CF, Chen LJ, Xiang LH, Zheng ZZ. Clinical analysis and etiology of porokeratosis. Exp Ther Med (2014) 8:737–41. 10.3892/etm.2014.1803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Errichetti E, Zalaudek I, Kittler H, Apalla Z, Argenziano G, Bakos R, et al. Standardization of dermoscopic terminology and basic dermoscopic parameters to evaluate in general dermatology (non-neoplastic dermatoses): an expert consensus on behalf of the International Dermoscopy Society. Br J Dermatol (2020) 182:454–67. 10.1111/bjd.18125 [DOI] [PubMed] [Google Scholar]
- 35. Zaballos P, Puig S, Malvehy J. Dermoscopy of disseminated superficial actinic porokeratosis. Arch Dermatol (2004) 140:1410. 10.1001/archderm.140.11.1410 [DOI] [PubMed] [Google Scholar]
- 36. Mazzeo M, Longo C, Manfreda V, Piana S, Bianchi L, Pellacani G, et al. Looking horizontally at disseminated superficial actinic porokeratosis: Correlations between in-vivo reflectance confocal microscopy and histopathology. Skin Res Technol (2020) 26:443–4. 10.1111/srt.12802 [DOI] [PubMed] [Google Scholar]
- 37. Leung AKC, Lam JM, Leong KF. Childhood Solitary Cutaneous Mastocytoma: Clinical Manifestations, Diagnosis, Evaluation, and Management. CurrPediatr Rev (2019) 15:42–6. 10.2174/1573396315666181120163952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Matito A, Azaña JM, Torrelo A, Alvarez-Twose I. Cutaneous Mastocytosis in Adults and Children: New Classification and Prognostic Factors. Immunol Allergy Clin North Am (2018) 38:351–63. 10.1016/j.iac.2018.04.001 [DOI] [PubMed] [Google Scholar]
- 39. Zhang G, Chen J, Liu X, Wang X. Concordance of reflectance confocal microscopy with histopathology in the diagnosis of mastocytosis: A prospective study. Skin Res Technol (2020) 26:319–21. 10.1111/srt.12779 [DOI] [PubMed] [Google Scholar]
- 40. Longo C, Zalaudek I, Argenziano G, Pellacani G. New directions in dermatopathology: in vivo confocal microscopy in clinical practice. Dermatol Clin (2012) 30:799–814, viii. 10.1016/j.det.2012.06.012 [DOI] [PubMed] [Google Scholar]
- 41. Scope A, Gill M, Benveuto-Andrade C, Halpern AC, Gonzalez S, Marghoob AA. Correlation of dermoscopy with in vivo reflectance confocal microscopy of streaks in melanocytic lesions. Arch Dermatol (2007) 143:727–34. 10.1001/archderm.143.6.727 [DOI] [PubMed] [Google Scholar]
- 42. Scope A, Benvenuto-Andrade C, Agero AL, Malvehy J, Puig S, Rajadhyaksha M, et al. In vivo reflectance confocal microscopy imaging of melanocytic skin lesions: consensus terminology glossary and illustrative images. J Am Acad Dermatol (2007) 57:644–58. 10.1016/j.jaad.2007.05.044 [DOI] [PubMed] [Google Scholar]
- 43. Cinotti E, Fiorani D, Labeille B, Gonzalez S, Debarbieux S, Agozzino M, et al. The integration of dermoscopy and reflectance confocal microscopy improves the diagnosis of lentigo maligna. J Eur Acad Dermatol Venereol (2019) 33:e372–4. 10.1111/jdv.15669 [DOI] [PubMed] [Google Scholar]
- 44. Palo S, Biligi DS. Utility of horizontal and vertical sections of scalp biopsies in various forms of primary alopecias. J Lab Phys (2018) 10:95–100. 10.4103/JLP.JLP_4_17 [DOI] [PMC free article] [PubMed] [Google Scholar]