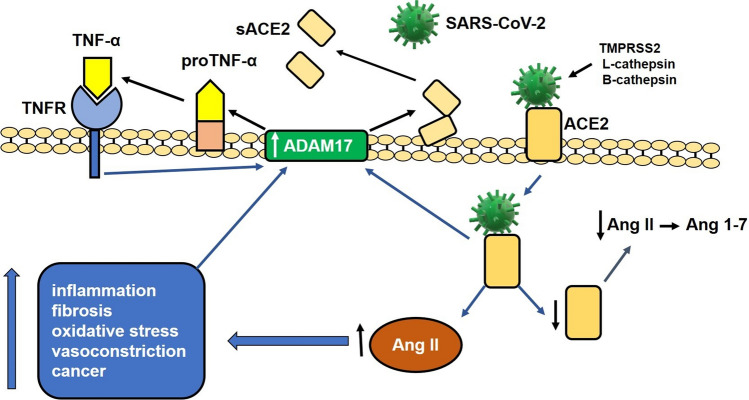
Fig. 1.
Involvement of the renin angiotensin system (RAS) in the pathogenesis of SARS-CoV-2 infection. The viral S protein binds to angiotensin I converting enzyme 2 (ACE2) after proteolytic cleavage by TMPRSS2 (serine transmembrane protease 2) and cathepsin L. The binding of the ACE2-cleaved S protein facilitates the entry of the virus into the cell and decreases the expression of ACE2 on the cell surface. The internalization of the virus/ACE2 complex decreases ACE2 levels and increases the activity of angiotensin II (Ang II) and the expression of ADAM17 (disintegrator and metalloproteinase 17) on the cell surface, which, when acting on ACE2, decreases the expression of this molecule on the cell surface. The increased activity of Ang II on the AT1 receptor induces the production of pro-inflammatory cytokines, oxidative stress (ROS), fibrosis, vasoconstriction, production of C-reactive protein (CRP), and increased activity of ADAM17. ADAM17 also acts on pro-TNF-alpha in the membrane, producing an active molecule that interacts with its receptor and induces the production of additional ADAM17. The activity of ADAM17 on ACE2 and the internalization of the virus/ACE2 complex reduce the amount of ACE2 on the cell surface and increase the amount of the soluble form of this molecule (sACE2) in the extracellular space. This process induces an increase in Ang II activity by inhibiting the conversion of Ang II to Ang 1-7, leading to a drastic increase in cytokine production with consequent deleterious effects