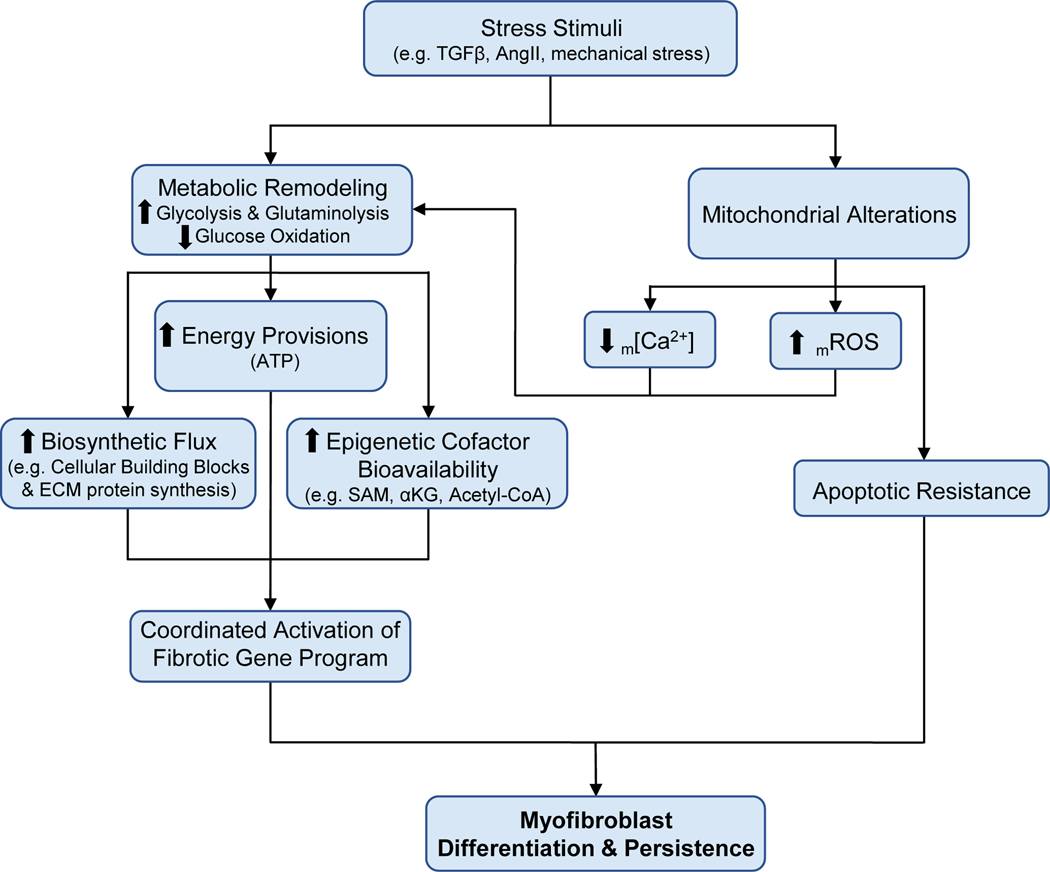
Figure 6. Working model of the influence of mitochondria and metabolism on myofibroblast differentiation and persistence.
Pro-fibrotic stress stimuli are critical for the activation and differentiation of resident cardiac fibroblasts to myofibroblasts. Pro-fibrotic stimulation results in significant metabolic remodeling that is critical for the energy provisions and synthesis of cellular building blocks required for proliferation, growth, and differentiation. Furthermore, these changes in cellular metabolism also regulate the bioavailability of metabolites which serve as cofactors for numerous epigenetic-modifying enzymes to coordinate the activation of the fibrotic gene program to promote differentiation. Recent work from our lab has shed new light on the mitochondria as a key regulator to this process by reducing mitochondrial Ca2+ uptake to promote the metabolic remodeling required for the differentiation process. The mitochondria also increase the generation of mROS to promote the necessary metabolic remodeling. Additionally, mitochondria under pro-fibrotic stimulation and in the terminally differentiated state downregulate the apoptotic pathways, promoting myofibroblast persistence which contributes to the progressive nature of cardiovascular disease. Collectively, the coordinated efforts of mitochondrial and metabolic remodeling are critical for the activation, differentiation, and persistence of myofibroblasts in cardiac disease, providing novel therapeutic targets to mitigate and potential reverse tissue fibrosis in disease. TGFβ – transforming growth factor beta; AngII – angiotensin II; m[Ca2+] - mitochondrial calcium concentration; mROS – mitochondrial-derived reactive oxygen species; ECM – extracellular matrix; SAM – S-adenosylmethionine; ⍺KG - ⍺-ketoglutarate.