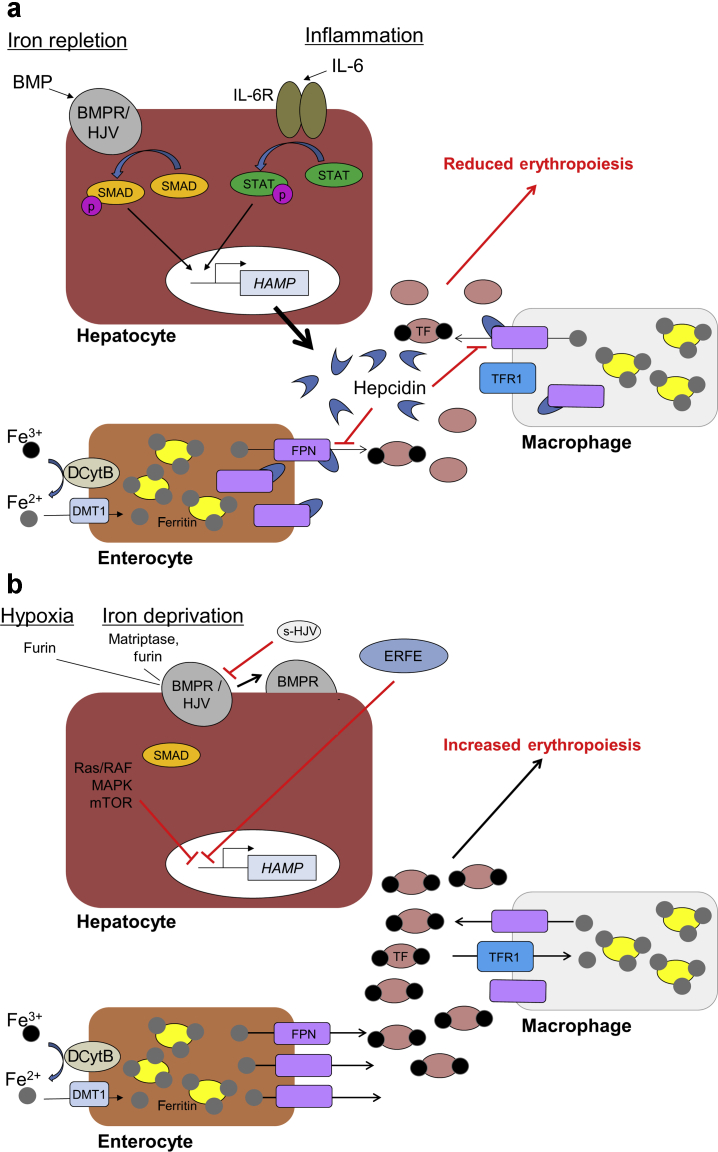
Figure 1.
Regulation of hepcidin and iron homeostasis. (a) Under conditions of iron repletion, bone morphogenetic protein (BMP) is upregulated. Binding of BMP to the BMP receptor (BMPR)/hemojuvelin (HJV) complex activates phosphorylation of Caenorhabditis elegans SMA/Drosophila mothers against decapentaplegic (SMAD), which then activates transcription of hepcidin antimicrobial peptide (HAMP) gene. Under inflammatory conditions, interleukin-6 (IL-6) activates the Janus kinase/signal transducer and activator of transcription (STAT) pathway that also activates transcription of HAMP. Hepcidin is then secreted by the liver and is able to bind ferroportin (FPN), a key exporter of iron into the circulation, on the surface of iron exporting cells such as macrophages and enterocytes. The binding of hepcidin to FPN results in the internalization and subsequent degradation of FPN, reducing iron export into the circulation. This then reduces the amount of transferrin-bound iron within the circulation, limiting the availability of iron for key biological processes, including erythropoiesis. (b) Under conditions of hypoxia or iron deprivation, transcription of HAMP is inhibited in hepatocytes via multiple mechanisms that are regulated by hypoxia-inducible factor, including furin-mediated conversion of membrane-bound HJV to soluble HJV (s-HJV), which can interfere with BMP signaling; erythroferrone (ERFE) produced during erythropoiesis signaling; and activation of the Ras rapidly accelerated fibrosarcoma mitogen-activated protein kinase (Ras/RAF-MAPK)–mammalian target of rapamycin (mTOR) signaling pathway. In the absence of hepcidin, iron is able to mobilize from cells to be used in heme formation during erythropoiesis. DCytB, duodenal cytochrome B; DMT1, divalent metal transporter 1; IL-6R, IL-6 receptor; TF, transferrin; TFR1, TF receptor 1.