Abstract
Background:
The VasQTM device was designed to improve the outcome of arteriovenous fistulae by optimizing the hemodynamics of the flow in the juxta-anastomotic region of the arteriovenous fistulae through tailored external support. The aim of the study was to evaluate the impact of the VasQ on outcome of radiocephalic arteriovenous fistulae in a real-world setting.
Methods:
This was a single-center, retrospective analysis of patients with either fistula creation before or after dialysis initiation with implantation of the VasQ device during creation of end-to-side radiocephalic arteriovenous fistulae between June 2018 and May 2019. The flow rate and vein diameter were evaluated intraoperatively, at discharge within 48 h postprocedure and at a follow-up of 1, 3, 6, 9, and 12 months.
Results:
Thirty-three VasQ devices were implanted during 33 radiocephalic arteriovenous fistula procedures. The study population comprised mostly of men, with an average age of 66 years. Mean intraoperative flow was 428 mL/min (range: 130–945). All patients were discharged with patent arteriovenous fistulae and mean fistula flow of 740 mL/min (range: 230–1300 mL/min). The primary patency was 100% and 79% at 3 and 6 months, respectively. Cumulative/secondary patency was 100% and 90% at 3 and 6 months, respectively.
Conclusion:
Data presented here suggest that the VasQ device has the potential to provide benefit to the functionality of radiocephalic arteriovenous fistulae.
Keywords: Radiocephalic arteriovenous fistula, arteriovenous fistula, external support device, VasQTM, hemodialysis, kidney disease, kidney failure
Introduction
Efficient hemodialysis is dependent on a reliable and long-lasting vascular access. Arteriovenous fistula (AVF) still remains the preferred type of access when possible, as it is considered more durable and associated with fewer complications and lower patient mortality.1,2 As such, it is recommended in many clinical practice guidelines.3–5 However, AVF utilization is hindered by relatively high primary failure rates, estimated to be 23% in a meta-analysis6 and reaching up to 70% in some studies, and by long maturation times7 as compared to arteriovenous grafts (AVGs). A significant benefit of AVF is higher patency rates than those reported with other modalities.8 However, especially over a longer time period, there is a constant decrease in patency, necessitating repeated interventions to maintain or reestablish it,6 creating a major impediment to vascular access. While the etiology of AVF-decreased patency is multi-faceted and complex, a prominent causative factor is thought to be the geometry9 of the anastomosis, that causes changes in hemodynamics of the juxta-anastomotic region (JAR). Such changes include turbulent blood flow, oscillating wall shear stress, and a considerable increase in radial forces with cyclic stretching of the intima and media.10 These cause intimal injury to which the endothelium responds with hyperplasia leading to stenosis and occlusion events.
The external support device VasQTM (Laminate Medical Technologies Ltd, Tel Aviv, Israel) was designed to improve AVF outcome by externally supporting the AVF and decreasing hemodynamic disturbance through optimization of the geometry of the JAR, therefore increasing wall shear stress and reducing the wall tension.11 In a previous randomized controlled study,12 VasQ was successfully implanted in patients during creation of the brachiocephalic arteriovenous fistula (BCAVF). The results showed that use of the VasQ device led to reduced rates of stenosis, larger vein diameter, and improved functional patency, without having any surgical complications or major differences in surgery duration compared to BCAVF creation without the device.
The 2006 National Kidney Foundation guideline on vascular access4 as well as the draft guideline issued in 20185 strongly recommend the radiocephalic arteriovenous fistula (RCAVF) as the first access of choice, if feasible. The choice of RCAVF preserves upstream veins for later use should the fistula fail and has the advantage of low rates of steal syndrome and rare ischemic monomelic neuropathy. However, these fistulae are characterized by poor maturation rates, mainly due to juxta-anastomotic stenosis.9,13 Thus, a device with a potential for reduction of stenosis can provide a meaningful benefit to creation of these types of fistulae.
Here, we present the results reporting our single-center experience of patients treated with the VasQ device in RCAVFs, with the aim of evaluating the performance of the VasQ implant in a real-world setting.
Methods
Patients and setting
Retrospective analysis of all records of RCAVFs created between October 2017 and May 2019 at the Asklepios Clinic Barmbek in Hamburg, Germany, was performed. All patients with simultaneous implantation of the VasQ device for RCAVFs were included in the present study, starting in June 2018 and until May 2019.
In accordance with local laws and regulations, no ethics committee or institutional review board approval is required due to the retrospective and anonymized nature of the study.
A thorough preoperative assessment of upper arm arterial and venous systems was performed by the main surgeon using ultrasound prior to every procedure. Eligibility criteria for the creation of RCAVFs and implantation of the device included a non-pathologic modified Allen test, vein inner diameter >2 mm using tourniquet, radial artery inner diameter >1.8 mm, triphasic arterial flow, and intact venous outflow.
Surgical procedure and outcome evaluation
All procedures were performed in a supine position with an extended arm fixed on an arm table under an axillary regional block. The radial artery and forearm cephalic vein were exposed and dissected in a typical manner. After an administration of 2000 international units of heparin, the artery and the vein were clamped. The distal end of the vein was ligated and divided. After a hydraulic dilation of the vein with heparinized saline, the artery and vein were measured using the disposable model selection tool (Figure 1; Laminate Medical Technologies Ltd), and the appropriately sized model of the VasQ device (1R, 2R, or 3R) was chosen. The VasQ device was then placed around the vein and held with a small bulldog clamp. An arteriotomy of approximately 6–7 mm was performed on the radial artery, and the cephalic vein was shortened to the required length with an angulation of approximately 40° and length of 6 mm. A side-to-end anastomosis was performed using a 7/0 monofilament Optilene® suture (B. Braun Melsungen AG, Melsungen, Germany). The device was fixed around the artery using a 5/0 Optilene® suture (Figure 1). After removing the clamps, intraoperative flow measurement was performed using either color-coded duplex sonography (Logiq S7; GE Healthcare, Chicago, IL, USA) in the brachial artery or transit time flow measurement (TTFM) (MiraQTM; Medistim, Oslo, Norway) around the vein. The wound was closed in a traditional manner layer wise.
Figure 1.
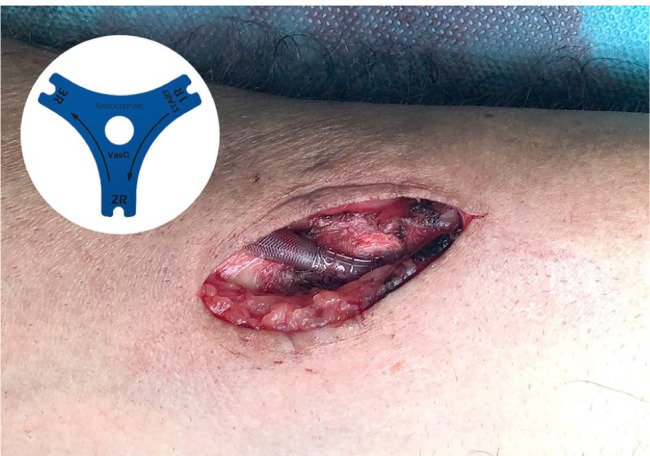
Disposable model selection tool and intraoperative image of the VasQ device in a radiocephalic arteriovenous fistula (RCAVF).
Volume flow was measured again within 48 h after surgery in the brachial artery using color-coded duplex sonography in most of the cases. The follow-up examinations were performed by the main surgeon after 1, 3, 6, 9, and 12 months postprocedure, if possible, and/or at an identification of any problems (e.g. stenosis, occlusion, cannulation difficulties, or dialysis issues) by the referring nephrologist. At the follow-up visit, patency and maturation were evaluated by clinical examination (auscultation and palpation of the AVFs (bruit, thrill), elevation test) by surgical staff to assess the access as well as by color-coded duplex sonography.
Data collection and definitions
In June 2019, demographic information as well as data on the current status of all AVFs was collected and included in the evaluations of the patency, functionality, and dialysis history of the access including all subsequent interventions.
Maturation was defined as an access suitable for hemodialysis and considered appropriate for cannulation with two needles and expected to deliver sufficient blood flow throughout the dialysis.14
Primary failure or early dialysis suitability failure was defined as an access that cannot be used by the third month following creation.14
Primary patency (PP) was defined as time from the initial dialysis access creation to the first reintervention for access dysfunction or thrombosis, the time of measurement of patency, or the time of its abandonment.14
Cumulative/secondary patency (SP) was defined as time from access creation until access abandonment (i.e. thrombosis or failed maturation) after one or more interventions or the time of measurement of patency including achievement of a censored event (death, lost to follow-up, transfer to peritoneal dialysis, or kidney transplantation).14
Primary functional patency was defined as the interval from the first cannulation of a newly created vascular access to the first reintervention to rescue the vascular access or to its abandonment.14
Interventions were defined as any surgical or endovascular treatment intended to maintain or reestablish patency.
Statistical analysis
Continuous variables are expressed as mean ± standard deviation or median (range). Categorical variables are expressed as numbers (percentage). Data were collected with a uniform data cutoff date of 20 May 2019 for the entire study population, and thus, the duration of follow-up varies between AVFs based on the date of implantation procedure. Since the patients underwent surgery at different time points, but retrospective analysis was performed on a set time, different time from surgery and follow-up is observed for each patient. Therefore, not all patients are included in 3- and 6-month analysis.
To address this variability, time-related outcomes, such as PP and SP, were estimated with Kaplan–Meier analysis and were compared with estimations calculated with the log rank test between the VasQ and control groups. Primary failure was compared using the chi-square test, and Poisson regression was used to test for difference in the rate of interventions per patient-years.
A p value < 0.05 was considered statistically significant. Statistical analyses were performed on the entire study population using JMP 13 software (SAS, Cary, North Carolina, USA) and Excel (Microsoft Corporation, Redmond, Washington, USA).
Results
Study population
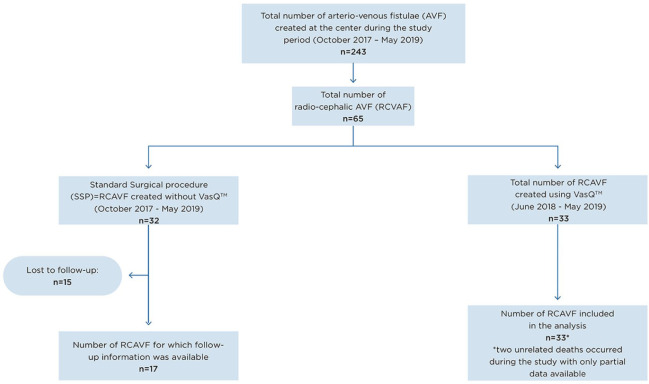
Between October 2017 and May 2019, 243 AVFs were created at our institution, of which 49 AVFs were created using the VasQ device: 16 upper arm and 33 forearm procedures. The latter were created in 32 patients (one patient had two RCAVFs with VasQ) with the first forearm device implanted in June 2018, and are included in the analysis. Thirty-two RCAVFs were created without VasQ, since the forearm device was either not available at that time or not suitable due to very sharp angulation between the radial artery and the cephalic vein in one case (Figure 2). The forearm VasQ study population comprised mostly men, with an average age of 66 years. The main reason for hemodialysis was end-stage renal disease (ESRD). Patients’ demographics as well as procedure-related data including surgical plan are presented in Table 1 (also see Table 2).
Figure 2.
CONSORT diagram for the study.
Table 1.
Demographic and clinical characteristics of study population.
| n | % | |
|---|---|---|
| Number of patients | 32 | |
| Number of RCAVF with VasQTM | 33 | |
| Gender: female | 11/32 | 34 |
| Average age in years (range) | 66 (28–87) | |
| Ethnicity | ||
| Caucasian | 28/32 | 88 |
| Arab | 2/32 | 6 |
| Asian | 2/32 | 6 |
| Main diagnosis | ||
| End-stage renal disease | 23/32 | 72 |
| Chronic kidney disease (preemptive) | 9/32 | 28 |
| Comorbidities | ||
| Diabetes | 6/32 | 19 |
| Hypertension | 4/32 | 13 |
| Coronary heart disease | 8/32 | 25 |
| Atrial fibrillation disease | 3/32 | 9 |
| Peripheral artery disease | 1/32 | 3 |
| Cardiomyopathy | 1/32 | 3 |
| Aortic insufficiency | 1/32 | 3 |
| Number of accesses (N = 33 with VasQ) | ||
| Access side: left | 31/33 | 94 |
| Previous failed ipsilateral vascular access | 11/33 | 33 |
| Snuff box AVF | 4/33 | 12 |
| RCAVF | 5/33 | 15 |
| BCAVF | 1/33 | 3 |
| EndoAVF | 1/33 | 3 |
| Tunneled central venous catheter | 19/33 | 58 |
AVF: arteriovenous fistula; RCAVF: radiocephalic arteriovenous fistula; BCAVF: brachiocephalic arteriovenous fistula; EndoAVF: endovascular arteriovenous fistula.
Table 2.
Postoperative outcomes.
| Follow-up time | Average 165 (range: 27–333) | Total 15.35 patient-years |
|---|---|---|
| Primary failure | 6% | 2/33 |
| Primary patency (total) | 79% | 26/33 |
| Secondary patency (total) | 88% | 29/33 |
| Functional patency (total) | 88% | 21/24 |
| Flow during surgery | 428 ± 202 mL/min. (33/33) | |
| Flow 1–2 days postprocedure | 740 ± 317 mL/min. (22/33) | |
| Flow at 1-month follow-up | 836 ± 312 mL/min. (21/33) | |
| Number of interventions | 4 | |
| Fistula successfully cannulated | 86% | 24/28a |
| Time from creation to cannulation (median) | 35 (29–99) For 20 patients of 24 cannulatedb |
|
5/33 patients were excluded, since not on active dialysis; 1/24 patient had successful maturation but still required an elevation due to the deep position of the forearm cephalic vein.
4 patients were not included in the above (three patients as those were revisions and veins were already matured at the time of procedure and were cannulated the next day, while one patient was not included as she refused to come to follow-up visits over 6 months).
Procedure outcomes
The procedures were performed technically successfully with implantation of the VasQ in all cases with no changes to the standard surgical procedure and no significant difference from standard surgery duration. Mean intraoperative flow was 428 mL/min (range: 130–945 mL/min). All patients were discharged with patent AVF and a mean fistula flow of 740 mL/min (range: 230–1300 mL/min). During the mean follow-up period of 165 days (range: 27–333 days), no device-related adverse events (e.g. site infection or perforation) were observed. Two unrelated death cases were reported during the study period.
Patency and maturation
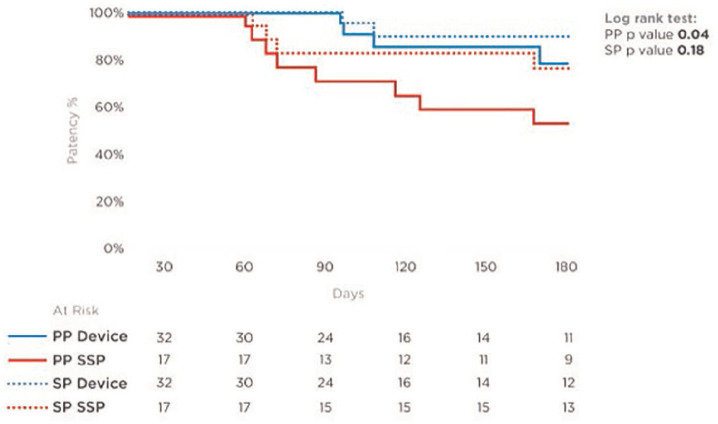
During the study period, PP of 79% (26/33) and cumulative/SP of 88% (29/33) were observed. Three accesses underwent successful balloon angioplasty (one of them underwent angioplasty twice, and the other two underwent one angioplasty each) due to >50% stenosis of the vein outside of the device area (one cranially of the device, one at the mid forearm, and one at the proximal forearm) and subsequent low flow after initially successful cannulations in all three patients. One patient referred to another hospital after 196 days with a reduced fistula flow of the RCAVF, in which a proximal elbow AVF was created and shortly after which a tunneled central venous catheter was implanted—due to failed maturation. In addition, three accesses were abandoned and converted to proximal accesses due to failed possibility of cannulations: one failed maturation due to anastomosis stenosis and initial intraoperative flow of 130 mL/min after 98 days in a patient with ESRD; one due to multiple debranching of the forearm cephalic vein and no possibility for cannulations after 109 days (both recorded as primary failure); and the third due to chronic obliteration of proximal forearm cephalic “outflow” vein after 217 days. From those, two had successful endovascular arteriovenous fistula (endoAVF) creations (one using Ellipsys® System (Avenu Medical, Inc, San Juan Capistrano, California, USA) and one using WavelinQ™ 4F System (Beckton, Dickinson and Company (BD), Franklin Lakes, New Jersey, USA)) and one underwent successful proximalization of the AV anastomosis to the middle forearm RCAVF using VasQ. Hence, PP was 100% and 79% at 3 and 6 months, respectively. SP was 100% and 90% at 3 and at 6 months, respectively (Figure 3 and Table 3).
Figure 3.
Kaplan–Meier analysis, demonstrating primary patency (PP) and secondary patency (SP) rates of both groups (with and without VasQ).
Table 3.
Comparison between study population and standard surgical procedure group.
| Standard surgical procedure | Study population | p value | |
|---|---|---|---|
| Follow-up | Total 19.22 patient-years | Total 15.35 patient-years | |
| Primary failure | 29.4% | 6% | 0.0251 |
| Primary patency (log rank, p value 0.04) | |||
| 3 months | 71% | 100% | |
| 6 months | 53% | 79% | |
| Secondary patency (log rank, p value 0.18) | |||
| 3 months | 82% | 100% | |
| 6 months | 76% | 90% | |
| Number of interventions | 12 | 4 | |
| Interventions per patient-year | 0.62 | 0.26 | 0.1029 |
For the entire group of patients, successful maturation was achieved in 88% (29/33) and cannulation was possible in 86% (24/28) of patients who were on dialysis either prior to or after the creation of AVFs.
The median creation-to-cannulation interval was 35 days (range: 29–99 days), although all analyzed fistulae exceeded the predefined 500 mL/min maturation flow rate within less than 30 days following surgery. One patient presented to the first follow-up 6 months after the initial procedure with difficulties of cannulations and underwent a successful percutaneous transluminal angioplasty (PTA) of middle forearm cephalic vein stenosis, which led to the first cannulation 99 days postcreation, since her dialysis unit did not follow up on her fistula for >3 months and used the catheter instead. Moreover, three accesses were created after failed distal AVFs with already prematured veins, and therefore, cannulations were possible on the first day after the creation. Those were not included in the calculation of average time to first use.
Comparison to the center’s standard of care
All consecutive RCAVF creations, performed without the VasQ by the current surgical team, were retrospectively reviewed for comparison of AVF outcomes. In total, 32 RCAVFs were created in the same manner as described above. All patients who attended at least one follow-up visit (17 of the 32) were included in the analysis, as 15 were lost to follow-up (Figure 2). This small group (standard surgical practice—SSP) had demographic and clinical characteristics similar to those of the study population: 35% females with mean age 62 years and 82% who had their access created already having ESRD and being on dialysis. Analysis of available data showed that AVFs created with the VasQ device had higher PP and SP at analyzed time points than those created without the device (Figure 3). The primary failure rate for AVFs with VasQ was significantly lower. Fewer interventions per patient per year were reported for AVFs created with VasQ than under SSP (VasQ 0.26 intervention per patient per year compared with 0.62 under SSP; Table 3), although without statistical difference in that small number of patients.
Discussion
In this retrospective study, we have evaluated the patency and functionality of RCAVFs created with the VasQ external support device in a real-world setting. RCAVF is the vascular access of choice that is recommended by guidelines.3,4 The advantages of this type of fistulae are lower rates of steal syndrome compared to upper arm brachial AVFs, minimal occurrence of ischemic monomelic neuropathy, and preservation of the option to create fistulae in more proximal locations.9 The primary failure rates reported here with the VasQ device are much lower than previously reported rates.7,15 These rates were also statistically lower than those which were observed in the comparative group treated under SSP in our center (6% with VasQ vs 29.4% under SSP: p = 0.0251). The PP that we observed (79% at 6 months) is higher than that reported by Siracuse et al.16 (48% for similar AVFs at 6 months). Our SP rate of 90% at 6 months is also higher than that observed by Lok et al.17 (50.5% at 6 months) with RCAVFs. Both PP and SP rates were higher using the VasQ than those in a comparative sample of RCAVFs created in our center and available for a follow-up without the use of the device (PP 53% at 6 months and SP 76% at 6 months), being statistically higher for PP in the VasQ group. Moreover, these rates are similar to those that have been observed with the use of the VasQ device in BCAVFs (Chemla et al.11 and Karydis et al.12). This is of note, as the RCAVFs have been known to be associated with lower patency and maturation rates than the BCAVFs.18
In the study population, only two primary failures (6%) were recorded, although they did not thrombose and remained patent before abandonment, as they had an insufficient draining vein flow. In light of the stringent parameters for fistula success, the low failure rate lends further support to the potential benefit offered by the VasQ device. Throughout the study period, four interventions were performed for the purpose of patency maintenance or reestablishment (0.26/patient year)—a lower than we observed in our SSP group (0.62/patient/year, although the difference was not statistically significant) and also lower than previously reported (0.63 angioplasties/patient/year19).
Importantly, no device- or procedure-related adverse events were observed in the reviewed patient data.
The duration of the maturation period between the establishment of the fistula and first cannulation is highly important. During this period, patients utilize alternatives for dialysis such as central vein catheters, that carry higher risks of infections20 and failure with or without thrombotic events.21 Thus, shortening the time to maturation has the potential of offering great benefit to patients. In the current study, the median time to first use was found to be 35 days (range: 29–99 days), shorter than that reported previously.22 A further positive indication is the fact that 2 days postprocedure, the flow was already over the threshold of 500 mL/min (mean 740 mL/min) and increased further to 836 mL/min at follow-up. It is important to note that this analysis does not include three accesses that were creations for failed distal AVFs with already prematured veins, allowing for cannulation 1 day after the creation. This subgroup was able to use the access immediately and avoid any alternative temporary access.
The study was limited by the retrospective design, by the limited data available for accesses created under SSP preventing its use as a control arm, and by the small sample size. However, the results are in line with those obtained in a randomized controlled study on BCAVFs (Karydis et al.12). By its very nature, analysis of real-world evidence provides valuable information about the performance of the device in the actual population and setting in which it is being used. Fistula functionality was assessed in patients under the routine care and monitoring of their doctors without significant resources required by participants of prospective clinical trials. Data presented here suggest that VasQ has the potential to increase functionality of RCAVFs. Future data from ongoing trials and market experience could further validate the potential of the device to improve RCAVF outcomes.
Acknowledgments
We thank Dr. Uri Heiman, DVM, from Tel Aviv University for his expertise and assistance throughout all aspects of our study and for his help in writing the manuscript.
Footnotes
Declaration of conflicting interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: R.S. received funds reimbursing for attending a related symposia and talk.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Robert Shahverdyan  https://orcid.org/0000-0002-8352-0954
https://orcid.org/0000-0002-8352-0954
References
- 1. Santoro D, Benedetto F, Mondello P, et al. Vascular access for hemodialysis: current perspectives. Int J Nephrol Renovasc Dis 2014; 7: 281–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dhingra RK, Young EW, Hulbert-Shearon TE, et al. Type of vascular access and mortality in U.S. hemodialysis patients. Kidney Int 2001; 60(4): 1443–1451. [DOI] [PubMed] [Google Scholar]
- 3. Fluck R, Kumwenda M. Renal association clinical practice guideline on vascular access for haemodialysis. Nephron Clin Pract 2011; 118(Suppl. 1): c225–c240. [DOI] [PubMed] [Google Scholar]
- 4. The National Kidney Foundation. KDOQI 2006 updates clinical practice guidelines and recommendations, 2006, https://www.kidney.org/sites/default/files/docs/12-50-0210_jag_dcp_guidelines-hd_oct06_sectiona_ofc.pdf
- 5. The National Kidney Foundation. KDOQI clinical practice guideline for vascular access: 2018, 2019, https://www.vasbi.org.uk/static/uploads/resources/kdoqi_vasc-access-review2019_v2.pdf [DOI] [PubMed] [Google Scholar]
- 6. Al-Jaishi AA, Oliver MJ, Thomas SM, et al. Patency rates of the arteriovenous fistula for hemodialysis: a systematic review and meta-analysis. Am J Kidney Dis 2014; 63(3): 464–478. [DOI] [PubMed] [Google Scholar]
- 7. Sheth RA, Freed R, Tavri S, et al. Nonmaturing fistulae: epidemiology, possible interventions, and outcomes. Tech Vasc Interv Radiol 2017; 20(1): 31–37. [DOI] [PubMed] [Google Scholar]
- 8. Perera GB, Mueller MP, Kubaska SM, et al. Superiority of autogenous arteriovenous hemodialysis access: maintenance of function with fewer secondary interventions. Ann Vasc Surg 2004; 18(1): 66–73. [DOI] [PubMed] [Google Scholar]
- 9. Quencer KB, Arici M. Arteriovenous fistulas and their characteristic sites of stenosis. Am J Roentgenol 2015; 205(4): 726–734. [DOI] [PubMed] [Google Scholar]
- 10. Mima A. Hemodialysis vascular access dysfunction: molecular mechanisms and treatment. Ther Apher Dial 2012; 16(4): 321–327. [DOI] [PubMed] [Google Scholar]
- 11. Chemla E, Velazquez CC, D’Abate F, et al. Arteriovenous fistula construction with the VasQTM external support device: a pilot study. J Vasc Access 2016; 17: 243–248. [DOI] [PubMed] [Google Scholar]
- 12. Karydis N, Bevis P, Beckitt T, et al. An implanted blood vessel support device for arteriovenous fistulas: a randomized controlled trial. Am J Kidney Dis 2020; 75(1): 45–53. [DOI] [PubMed] [Google Scholar]
- 13. Lee T, Roy-Chaudhury P. Advances and new frontiers in the pathophysiology of venous neointimal hyperplasia and dialysis access stenosis. Adv Chronic Kidney Dis 2009; 16(5): 329–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schmidli J, Widmer MK, Basile C, et al. Vascular access: 2018 clinical practice guidelines of the European Society for Vascular Surgery (ESVS). Eur J Vasc Endovasc Surg 2018; 55(6): 757–818. [DOI] [PubMed] [Google Scholar]
- 15. Miller PE, Tolwani A, Luscy CP, et al. Predictors of adequacy of arteriovenous fistulas in hemodialysis patients. Kidney Int 1999; 56(1): 275–280. [DOI] [PubMed] [Google Scholar]
- 16. Siracuse JJ, Cheng TW, Arinze NV, et al. Snuffbox arteriovenous fistulas have similar outcomes and patency as wrist arteriovenous fistulas. J Vasc Surg 2019; 70(2): 554–561. [DOI] [PubMed] [Google Scholar]
- 17. Lok CE, Sontrop JM, Tomlinson G, et al. Cumulative patency of contemporary fistulas versus grafts (2000-2010). Clin J Am Soc Nephrol 2013; 8(5): 810–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jeong S, Kwon H, Chang JW, et al. Patency rates of arteriovenous fistulas created before versus after hemodialysis initiation. PLoS ONE 2019; 14(1): e0211296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Al-Balas A, Lee T, Young CJ, et al. The clinical and economic effect of vascular access selection in patients initiating hemodialysis with a catheter. J Am Soc Nephrol 2017; 28(12): 3679–3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kumbar L, Yee J. Current concepts in hemodialysis vascular access infections. Adv Chronic Kidney Dis 2019; 26(1): 16–22. [DOI] [PubMed] [Google Scholar]
- 21. Gunawansa N, Sudusinghe DH, Wijayaratne DR. Hemodialysis catheter-related central venous thrombosis: clinical approach to evaluation and management. Ann Vasc Surg 2018; 51: 298–305. [DOI] [PubMed] [Google Scholar]
- 22. Allon M, Imrey PB, Cheung AK, et al. Relationships between clinical processes and arteriovenous fistula cannulation and maturation: a multicenter prospective cohort study. Am J Kidney Dis 2018; 71(5): 677–689. [DOI] [PMC free article] [PubMed] [Google Scholar]