Abstract
Recently, the potential dangers of viral infection transmission through water and air have become the focus of worldwide attention, via the spread of COVID-19 pandemic. The occurrence of large-scale outbreaks of dangerous infections caused by unknown pathogens and the isolation of new pandemic strains require the development of improved methods of viruses’ inactivation. Viruses are not stable self-sustaining living organisms and are rapidly inactivated on isolated surfaces. However, water resources and air can participate in the pathogens’ diffusion, stabilization, and transmission. Viruses inactivation and elimination by adsorption are relevant since they can represent an effective and low-cost method to treat fluids, and hence limit the spread of pathogen agents. This review analyzed the interaction between viruses and carbon-based, oxide-based, porous materials and biological materials (e.g., sulfated polysaccharides and cyclodextrins). It will be shown that these adsorbents can play a relevant role in the viruses removal where water and air purification mostly occurring via electrostatic interactions. However, a clear systematic vision of the correlation between the surface potential and the adsorption capacity of the different filters is still lacking and should be provided to achieve a better comprehension of the global phenomenon. The rationalization of the adsorption capacity may be achieved through a proper physico-chemical characterization of new adsorbents, including molecular modeling and simulations, also considering the adsorption of virus-like particles on their surface. As a most timely perspective, the results on this review present potential solutions to investigate coronaviruses and specifically SARS-CoV-2, responsible of the COVID-19 pandemic, whose spread can be limited by the efficient disinfection and purification of closed-spaces air and urban waters.
Keywords: Coronavirus, SARS, Wastewater, Porous, Carbon, MOF
1. Introduction
Viruses are ubiquitous in nature and consequently their interactions with superior organism and human beings are constant. Overall, the genetic diversity of viruses, their capabilities to change and adapt, as well as their significant presence in nature are amazing. It has been estimated that the total number of viral particles is significantly higher than the number of all cells of all the organisms on Earth combined [1]. A virus is defined as a non-cellular infectious agent, and the fundamental question whether they may or not be classified as living organisms is still debated. Today, only 6,000 viruses are known while several millions viral strains exist but are unknown [2]. The extreme diversity of the viral populations is also related to the problems encountered in viruses’ eradication and to the various public health threats. Indeed, viruses are particularly difficult to eliminate because they are continuous evolving under natural selection pressure, allowing them to counteract the protection offered by vaccines or specifically targeted drugs. Viral particles cannot subsist independently, and the first step of the diffusion is the infection of a cell, that is invaded with the viral genomic and proteic material.
Almost all known viruses have their own specific target in a living organism, which implies the existence of a specific receptor on the cell surface where the virus attaches itself to gain entry to the cell [2], the preferred viral receptors also determine the cell types that will most probably suffer from the infection. Once inside the cell the viral genome is replicated and translated, also exploiting the cellular machinery, resulting in the production and maturation of novel viral particles that can be expelled from the cell, usually killing the latter in the process, and contribute to the further diffusion of the agent. Viral transmission is mostly achieved by the expulsion of viral particle with bodily fluid or respiration and the subsequent contact with other organisms. Normally, and once again due to the receptor specificity, the viral transmission is mostly happening between organisms of the same, or closely related, species. However, the virus mutation capability can also lead to zoonosis, i.e. overcoming the interspecies barrier greatly facilitating the transmissibility and contagious capacity. Indeed, zoonosis is usually recognized as a crucial step in the early development of uncontrolled epidemics and outbreaks.
It is nowadays well established that viruses may represent a serious threat to public health, since they can be extremely pathogenic and be at the origin of extremely dangerous diseases [1], [3], [4]. Indeed, since ancient times mankind had to cope with the diffusion of epidemic and pandemic, whose periodic outbreaks have marked many historical periods. The constant threat of viral-based diseases is also witnessed by the fact that in many ancient cultures its spreading was attributed to the angers of Gods, see for instance Sekhmet in ancient Egypt or Artemis in classical Greece. Viral-based diseases may indeed severely affect different organs, and their spread is favored by the possibility of human-to-human contagion, that is determined by the inherent viral infectivity rate.
Every year, humanity is facing challenges with new types of viruses that threaten the human health and can cause epidemics and, in the worse scenario, pandemics [2], the most common, and relatively innocent one being seasonal flu. A more dangerous example is constituted by the Middle East respiratory coronavirus syndrome (MERS coronavirus) appeared in the spring of 2015 in South Korea. The outbreak took the South Korean authorities by surprise and forced them to take urgent epidemiological measures [2]. MERS mortality ratio amounts to more than 35% and, as stated in the World Health Organization (WHO) Newsletter, “there is currently no specific treatment or vaccine for this disease”. This example alone justify the extreme importance and interest devoted to the research on viruses and its vital importance to maintain healthy environments for the society [5].
In 2020, humanity is facing new global social and economic challenges induced by the apparition of a novel coronavirus in late 2019. The first cases of an unknown coronavirus were recorded on 31th December in the Chinese city of Wuhan, which has a population of nearly 12 million [6]. The effects due to this disease were rapidly observed in mainland China neighboring countries: Thailand, Japan, South Korea and Taiwan [7]. Since then, the virus dissemination has continued, with the epicenter moving from Asia, Europe and later Americas, the new coronavirus was later named severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) to differentiate it from the original SARS-CoV appeared in 2003, while the associated disease has been styled as COVID-19. On March 11th 2020, WHO declared COVID-19 a pandemic [7]. As late as November 18th 2020, more than 55 million people have been infected worldwide, new daily cases and deaths are still globally increasing, although the original Asian hot-spots seem under relative control. COVID-19 affects the respiratory system, the gastrointestinal tract or the nervous system; it can cause bronchitis, pneumonia and, in severe cases, death [1], [5], [6]. SARS-CoV-2 originally developed in animals, probably pangolins, which acted as the initial main carriers. However, through zoonosis, the transmissibility via human-to-human contact became possible and represent today the main contagious route [4], while recent studies have also pointed to the possible airborne transmission of this virus via aerosols [8]. The mortality ratio of SARS-CoV-2 is relatively low, especially when compared with SARS-CoV or MERS, and is estimated to not exceed 1–2%, severe complications and death usually appearing in aged patients or in subject presenting significant comorbidity, such as diabetes or hypertension. However, the transmissibility and diffusivity of the virus are extremely high, and are also facilitated by the presence of asymptomatic, or barely symptomatic carriers contributing to its spreading.
COVID-19 pandemic and the effects to the related containing measures on the different aspects of the daily life have been clearly highlighted, also towards the general public, the importance of developing new strategies to minimize the corresponding infection risks via the reduction of exposure routes for this and other viruses and to generate healthy environments. The quantification of coronavirus particles in wastewater is indeed an interesting and original approach to the study of the spread of the pathogens [9], since SARS-CoV-2 particles have been found in wastewater in a quantity correlating well with the epidemic growing ratio [10], pushing many countries to begin a more systematic wastewater monitoring [11]. Indeed, the increases of the number of SARS-CoV-2 particles in wastewater occur even before COVID-19 symptoms appear in patients, hence its monitoring may allow the early detection of possible epidemic outbreaks [12]. Even if interesting such an approach requires the use of concentration method to overcome the low concentration of viral material and it relies on the use of adsorbents such as anion exchange resin [13].
It is known that the dissemination of viruses can take place by different routes including direct contact with infected animals and humans, or through dispersion of viral particles in water and air [3], [14], [15], [16]. As a consequence, it is necessary to establish high-quality water and air treatment to contribute to the reduction of virus dissemination, lowering the infectivity and transmissibility ratio, and hence preventing serious epidemic outbreaks [17], [18]. Thus, to be effective and to respect the public-health standards, purification of water and air should not be restricted to the mechanical elimination of large particles, but should also include the removal of biological pollutants like viruses and bacteria [19], [20], [21], [22]. Indeed, infections can be transmitted via the diffusion of viral particles by air-droplets, through moist soil, surfaces, and water [23]. The diffusion of viral material in household occupied by infected persons is of particular concern. In some instances, infection can be propagated by the ingestion of contaminated food, such as vegetable or fruits, or by drinking non-sanitized water. Viruses may reach the surfaces of fruits and vegetables when they are fertilized with non-disinfected wastewater. If the contamination through infected water and food is at the moment well managed in developed countries, the more difficult access to alimentary resources in developing or underdeveloped areas still represents a serious public-health issue, seriously undermining human health development.
Furthermore, the quantification of virus content, i.e. the precise determination of the type and concentration of virus is far from being an innocent subject, and many important scientific breakthroughs have been realized in the last years. Virus quantification is fundamental not only for the analysis and purification of effluent or wastewater but also for the production of viral vaccine, antiviral agents, or recombinant proteins. Historically, the more common virus quantification methods included plaque titer assay, fluorescent focus assay (FFA), and 50% tissue culture infection dose (TCID50). Without entering into too much details, that will be out of the scope of the present review, those methods are based on microbiology techniques in which hosts cells are exposed to viral samples and the number of infected cells that will lyse eventually identified by immunostaining, is recorded giving an indication of the virus quantity. The actual quantification in this case is achieved providing either the number of plaques formed by the lysed cells, or of the stained foci, expressed per unit of sample and volume, or by its logarithm (log10). Conversely, TCID50, that yields the amount of virus needed to lyse half of the host cell, is mostly used in clinical frameworks. More recently, direct measures of the amount of viral material have emerged testing either specific viral protein or its genetic material. In particular, quantitative polymerase chain reaction (qPCR) is used to amplify and detect the amount of viral DNA or RNA, while enzyme-linked immunosorbent assays (ELISA) are sensible to the viral proteins and acts through an antigenic mechanism. It is worthwhile to mention that the use of transmission electron microscopy (TEM) may allow to quantify some virus via direct visualization. In this case, the quantity of virus is expressed directly as the quantity of viral material, or particles, per unit of sample and volume. Modern methods such as qPCR or antigenic tests are much faster than the classical cellular based techniques, and have the advantage to directly target the viral material, although they may be subjected in some instance to contamination [17], [24].
The presence of viruses in air and water can be faced using different technologies with a varied degree of sophistication, operational costs, removal efficiency and energy consumption. These technologies comprise mainly mechanical and electrical filtration using for example ceramic filters [25], [26], [27], coagulations processes [28], saturated soil column [29], reclamation systems [30], [31], [32], membrane bioreactor process [33], photocatalytic disinfection [34], [35], concentration methods [36], ozone generators and UV irradiation [37], chemical disinfection and membrane-based processes [38]. Among these technologies, membrane-based processes based on adsorption appear as a low cost and efficient technique.
Different membranes have been proposed for virus removal from water and air: ultrafiltration [39], [40], nanofiltration [41] and reverse osmosis membranes [20], [42], [43], [44], [45]. Nowadays, a large variety of materials has been proposed to this aim, also exploiting specific interactions with the viral capsid. They include activated carbon [46], [47], [48], [49], polysaccharide (PS)-based materials [50], kaolinite and fiberglass [51], Cu and Ag compounds [52], [53], quartz sand [54], functionalized chitosan nanofibers [55], hydrochar [56], nano-TiO2 [57], resins [13], poly(ethylenimine) [58], Fe/Ni nanoparticles [59] and zeolites [60]. Alternative strategies for fluid decontamination can rely on the treatment of wastewaters with natural occurring or biocompatible organic compounds, such as carbohydrates, that show high antiviral effect coupled with the absence of unwanted side effects such as cytotoxicity. Note that the majority of these studies was focused on the removal of the viruses from water but the results can be extended and used as a basis to develop purification methods for air.
The present review will focus on the different strategies for the rational design of adsorbents materials that can be easily implemented to eliminate viral particles from air or liquid fluids. After describing in details the structure of viruses, this review will be organized in sections corresponding to the main classes of adsorbents: carbon-based materials, oxide-based materials, zeolites, silica, metal–organic frameworks, clays and carbohydrates. We will present and critically comment the available practical technologies for removing viruses by adsorption and our perspectives will be provided in the last section of this review.
2. Description of virus structures
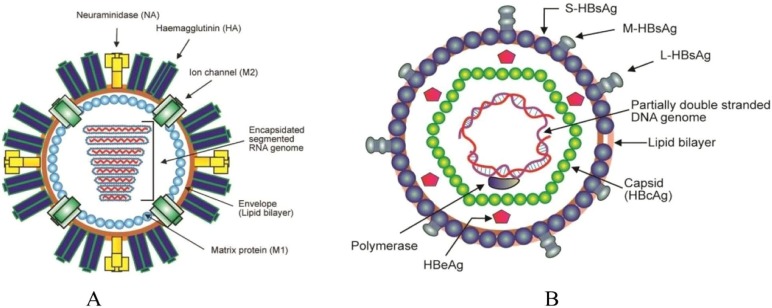
In addition, to the possible threats to public health system and social organizations, virus biology is also complex and fascinating. By a general definition, viruses are small microorganisms that do not possess a cellular structure. Overall, the classification of viruses may be diverse and rooted either on molecular biology or phenomenological and phenotypical factors such as: 1) the type of nucleic acid encoding the genetic information, 2) its structure (single- or double-chain, linear, circular, fragmented, unregimented), 3) the structure, dimensions, type of symmetry and number of capsomers (i.e. the subunits constituting the virus external shell, termed capsid, and mainly constituted of proteins), 4) the presence or absence of the outer shell (super capsid), 5) the antigenic structure, 6) the genetic interactions taking place, 7) the geographical distribution, 8) the route of infection transmission and 9) the type of host (animal, plant, bacteria) [4], [61], [62]. Based on the literature, the best known kind of viruses are: rotaviruses, adenoviruses, hepatitis type A, B, C, poliomyelitis (poliovirus) [25] and coronaviruses. The size of the virus structural units (virions) range from 20 to 300 nm [20]. The composition of virions includes nucleic acids, either ribonucleic (RNA) or deoxyribonucleic (DNA) acids, enclosed by a membrane and a protein shell constituting the capsid, see Fig. 1 . Protein capsid protects and encloses the viral genome encoded by the nucleic acids. Thus, protein capsid and nucleic acids are globally known as nucleocapsid. Note that at a microscopic level the capsid, and hence viruses, may assume different shapes: cubic, spherical, rod-shaped, etc. [62]. The specific shape of the capsid and the structural proteins defining the interactions with the cellular receptors will determine on the one side the type of organisms and cells that can be infected by a given virus, and on the other side the transmissibility and the possibilities for the interactions with external materials. For instance, small size viruses can be diffused further with fluid emission and through air compared to heavier and bigger structures.
Fig. 1.
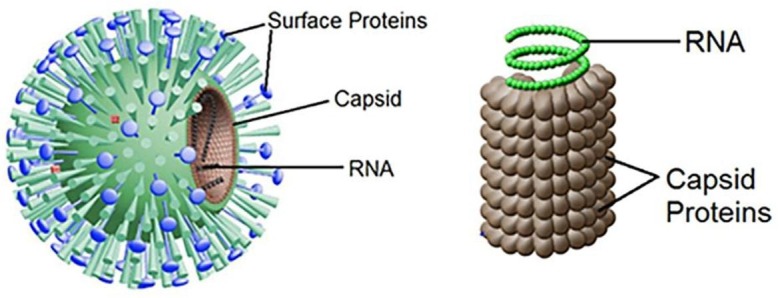
Illustration of a virus structure.
The most widespread classification of viruses depends on the nature and structure of their genome rather than on the diseases that they can cause [3]. As a consequence, a basic difference exists between DNA and RNA viruses, which may have single or double chains of genetic material, see Fig. 2 . Furthermore, single chain RNA viruses are divided into RNAs with positive polarity and RNAs with negative polarity [3]. Typically, DNA viruses replicate in the nucleus of the host cell, while the replication process of RNA viruses takes place in the cytoplasm. At the same time, some positive-polarity single-chain RNA viruses, called retroviruses, although replicating in the cytoplasm, use a completely different strategy involving the preliminary retro-transcription of RNA into DNA performed by the viral retrotranscriptase.
Fig. 2.
Schematic illustration of A) influenza A virus (i.e., RNA virus) and B) hepatitis B virus (i.e., DNA virus) [4].
A further difference in viral strains can be related to the structural properties of the virions leading to the definition of simple and complex particles [3].
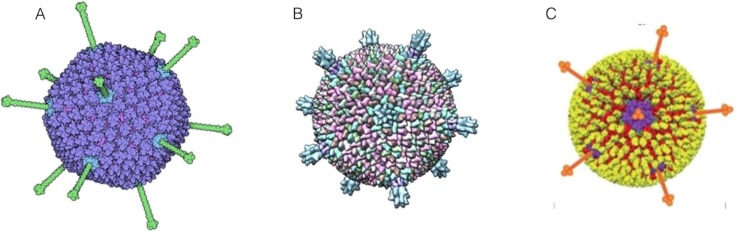
Simple viruses are constituted by an external shell, named capsid, formed by the specific external proteins named capsomers, and whose shape determines the virus symmetry. Complex viruses have an additional outer shell, the supercapsid, located on top of the capsid. The supercapsid contains the inner protein layer, formed by the M−protein, and a more bulky layer of lipids and carbohydrates, that is formed by molecular constituents extracted from the host cell membranes [4]. In addition, viral glycoproteins penetrate into the supercapsid resulting in curly protrusions (spikes, fibers) and act as receptor allowing the interaction with cell membrane proteins and hence the infection [3]. Examples of different classes of viruses are illustrated on Fig. 3 .
Fig. 3.
Examples of viruses: A) Adenovirus, B) Sulfolobus turreted icosahedral virus I and C) Mammalian orthoreo virus [62].
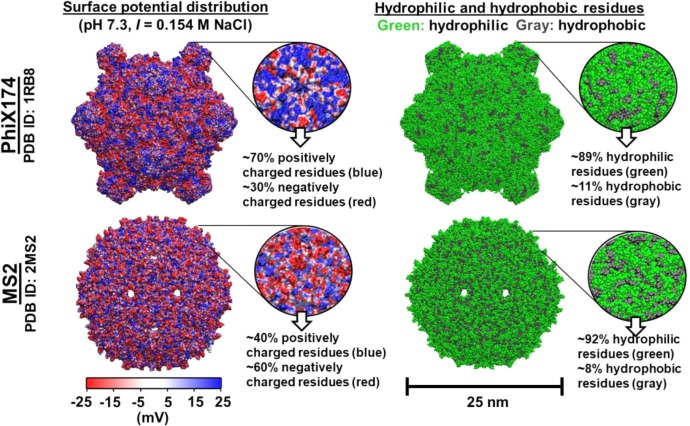
Although viral particles may, on a first approximation, be regarded as structured objects, traditional separation methods like adsorption and capture have shown a generally low efficiency, when used in practical applications. Probably, the reason for this lack of performance may be associated to the specific physicochemical and biochemical properties of viral particles, namely, their low diffusion mobility, that can be traced back to their molecular size, and the complex multicomponent and rather labile structure of the virions [63], [64]. Indeed, the structural properties of the virus’ surface determine the mechanism of their interactions with the adsorbent surface, its bio-affinity, and ultimately the adsorption rate, which is usually accompanied by the formation of highly specific (or bio-affinity) ionic, hydrophobic and coordination bonds [25], [65], [66]. Thus, the physicochemical properties of viruses such as their size, morphology, surface charge, capsid conformation and the distribution of charged, hydrophilic, and hydrophobic amino acids strongly affect the virus-material interaction [67], [68] as schematically reported in Fig. 4 .
Fig. 4.
Capsid structure and hydrophilic/hydrophobic amino acid distribution of MS2 and PhiX174 phages (bacteriophage). Adapted from [67] with permission from Elsevier.
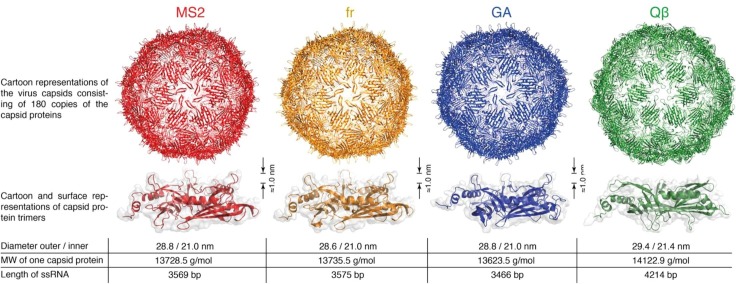
Hence, the study, also at molecular level, of the specific interplay between the adsorption processes and the specific nature of the viral envelope is essential for the development and the improvement of separation methods and strategies for viruses capture and elimination. In addition to structural optimization, mainly based on the adaptation of the adsorbent material to the virus size [3], surface charged material could be used to exploit electrostatic effects to enhance viruses adsorption and inactivation [26], [69], [70]. Some specific viral particles have been extensively used to model the adsorption of viruses since they permit a sufficiently large representation of the different structural and physico-chemical space spanned by the different pathogens and, as reported in Fig. 5 , they include the bacteriophages male specific type 2 (MS2), fr, GA and Qβ.
Fig. 5.
Structures of most common virus-like particles – bacteriophages MS2, fr, GA and Qβ utilized as models in adsorption experiments. Reprinted with permission from [66].
3. Carbon-based materials
Carbon-based materials are the most used adsorbents for water and air treatment and, consequently, they have been also largely applied in virus removal. A summary of different carbon-based materials used for virus adsorption is reported in Table 1 and critically analyzed in the following. Two types of activated carbon, conventional granular activated carbon and an activated carbon fiber composite, have been tested and applied for virus removal from water [48]. The raw material was characterized by a rigid mass of interlocked fibers of an average length of 0.1–0.4 mm and an average width comprised between 5 and 100 µm. The bacteriophage MS2 (having a radius of approximatively 25 nm) was chosen as a model for this study. It has to be noted that bacteriophage is easier and cheaper to grow and test, especially compared to viral particles and, consequently, it is often used as a model virus especially to study water sanitation. It was shown that the shape of activated carbon could either inhibit or enhance the removal of the large bacteriophage particles. Indeed, the study confirmed that carbon fiber composite having a lower total area (840 m2/g) was more efficient for virus adsorption than granular activated carbon with larger total area (1050 m2/g) resulting in a higher virus removal due to different shape and size fraction of the activated carbons.
Table 1.
Summary of some advantages and limitations of carbon-based materials used for virus inactivation.
| Adsorbents | Type of virus | Consequences and advantages |
|---|---|---|
|
|
|
|
|
|
|
|
|
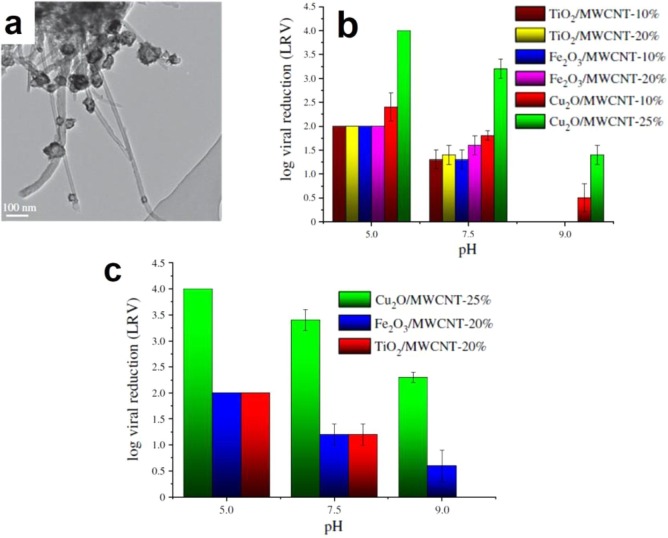
Matsushita et al. [47] investigated the removal of the two bacteriophages Qβ (diameter 23.5 ± 0.8 nm) and MS2 (diameter 22.5 ± 1.0 nm) by adsorption on commercially available powdered activated carbon (N-PAC) and super-powdered activated carbon (S-PAC). This study allowed to pinpoint some crucial factors for virus removal, namely: (i) increasing the electrophoretic repulsive force between virus and particles of powdered activated carbon results in the decreasing of virus removal, (ii) the increase of N-PAC pore volume improved the virus removal, a pore diameter of 20–50 nm was shown to be necessary to trigger adsorption, (iii) the increase in hydrophobicity of the virus surface results in a higher removal, and (iv) the low negative surface charge of AC surface enhanced the virus removal. Similar findings were obtained by Nemeth et al. [72] who proposed multi-walled carbon nanotubes (MWCNT) sensitized by Cu2O, TiO2 and Fe2O3 NPs in order to build hybrid membranes for MS2 bacteriophages removal from contaminated water. Cu2O-coated MWCNT membrane (see Fig. 6 a) showed the highest activity in virus removal in the pH range comprised between 5.0 and 9.0, yielding a 4-Log, i.e. 99.99% lower bound, for the percentage of virus retention, and, consequently, were considered promising for virion uptake (Fig. 6b and 6c).
Fig. 6.
(a) TEM micrograph of Cu2O/MWCNT nanocomposite membrane with 25% of MWCNT, (b) MS2 bacteriophage removal using MWCNT-based nanocomposite membranes in batch experiments and (c) MS2 bacteriophage removal using MWCNT-based nanocomposite membranes in flow experiments. .
Adapted from [72]
4. Oxide-based materials
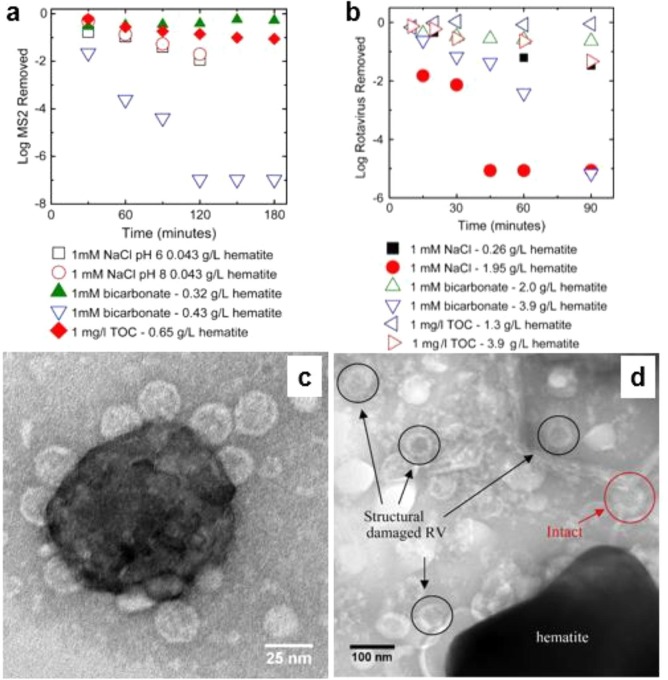
It is known that viral capsid surfaces are negatively charged, hence surface positively charged nanoparticles may be well suited for virus removal through electrostatic interaction, at least at suitable pH [73]. Indeed, electrostatic-mediated adsorption inactivates viruses and can be used both for water and air disinfection. Table 2 provides a summary of oxide-based materials employed to remove or inactivate viruses. Mazurkow et al. [71] reported spray-dried alumina granules modified with copper (Cu) and copper oxide (Cu2O) nanoparticles (NP) as effective adsorbents against viruses, thanks to the capacity of the nanoparticles to create a high density of adsorption sites. The experiments were conducted in flow reactor and showed a removal of 99.9% of the MS2 bacteriophages by Cu2O and Cu, conversely CuO had no impact on virus removal. Gutierrez et al. [73] performed batch and dynamic experiments in order to investigate the removal of group A porcine rotavirus (whose diameter is of 74.57 ± 1.32 nm) and bacteriophage MS2 (diameter of 25.42 ± 0.93 nm) using glass fiber coated with hematite Fe2O3 NPs having a surface area of 80.75 m2/g and total pore volume of 0.0835 cm3/g. The batch mode disinfection demonstrated a high removal of both rotavirus and bacteriophage MS2 quantified by 2.49 × 1011 plaque forming unit/g and 8.9 × 106 focal forming unit/g, respectively (Fig. 7 a and 7b), while the concentration of hematite used was 0.043 and 0.26 g/L for virus and bacteriophage MS2, respectively. Transmission electron microscopy (TEM) images confirmed the electrostatic adsorption of MS2, while they also underlined structural damages to the rotavirus caused by the interaction with hematite that also participate in the virus inactivation. (Fig. 7c and 7d). While hematite NPs present the advantage of offering more available adsorption active sites coupled with strong electrostatic attraction, which can be beneficial in favoring virus depletion, it was concluded that the inactivation of viral particle might depend on the specific proteins of its capsids or the robustness of its structure [73], and hence its efficacy could vary considerably between different viral strains. However, it is clear that hematite NPs may represent a most valuable system to achieve virus removal from contaminated water and its use could be extended to air treatment.
Table 2.
Summary of some advantages and limitations of oxide-based materials used for virus inactivation.
| Adsorbents | Type of virus | Consequences and advantages |
|---|---|---|
|
|
|
|
|
|
| Magnesium oxyhydroxide |
|
|
|
|
|
| Nano-TiO2-membranes;Spherical Fe/NiNPs |
|
|
| Silica-decorated TiO2 NPs |
|
|
Fig. 7.
Removal of MS2 and rotavirus onto hematite NPs surface: (a) MS2 removal by hematite NPs; (b) rotavirus removal by hematite NPs; (c) TEM image of MS2 bacteriophage adsorbed onto hematite NPs surface; (d) TEM image of structurally damaged rotavirus particles contacted with hematite NPs surface. Reprinted from [73].
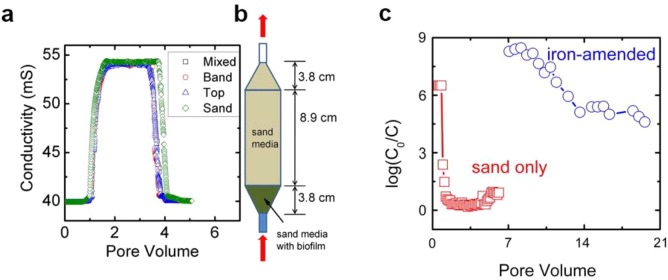
Similarly, Bradley et al. [74] have studied the potential application of a biosand filter filled with iron oxide NPs to remove virus, see Fig. 8 a and 8b. The iron oxides were obtained as result of zero-valent iron particles corrosion, while the purification experiments were performed in continuous flow process and showed a significantly higher removal of bacteriophage MS2 by an iron-sensitized sand column (5log10) compared to a sand-only column (0.5log10) (Fig. 8c). The removal mechanism was again explained by the electrostatic interaction of negatively charged MS2 particles with the positively charged iron oxides. In this same line of investigation, Domagala et al. [75] used Cu2O/MWCNTs filters to remove MS2, finding that while the filters were efficient, electrostatic interactions should not be held as the sole responsible of the adsorption [75].
Fig. 8.
(a) Breakthrough curves of NaCl tracer from glass columns packed with sand and zero-valent iron; (b) small-scale glass columns packed with iron oxides used for virus removal; (c) the log10 reductions bacteriophage MS2 obtained by continuous flow through clean quartz sand with no iron particles and sand mixed with 10% (vol.) iron. Reprinted from [74].
Michen et al. [26] have proposed the use of magnesium oxyhydroxide, embedded into a ceramic filter (10, 15, and 20% of MgO), to improve viruses removal from water. These filters showed an enhanced removal of about 4-log of bacteriophages MS2 and PhiX174. Once again, the enhanced removal of the virus was attributed to the establishment of favorable electrostatic interactions between the negatively charged virion particles and the positively charged patches of magnesium oxyhydroxides. Furthermore, it was also concluded that viruses may be inactivated in an alkaline environment and the presence of the slightly soluble Mg(OH)2 can indeed cause the increase of water pH above 9. Albeit efficient for viral decontamination, the high increase of pH alters the physico-chemical properties of water and, in particular, makes it not suitable for drinking purposes; hence, a continuous flow mode was proposed for use of the ceramic filters. Globally, the results suggested that magnesium oxyhydroxide could be a good adsorbent for viruses also due to its availability, high isoelectric point and low cost.
The finding that viruses can be attracted by positively charged surface has been confirmed by Wegmann et al. [42], in analyzing the impact of electropositive zirconium (hydr)oxide NPs coatings on the performance of ceramic filters to remove viruses from water. Indeed, the internal filter surface was coated by ZrO2 nanopowder via dip-coating and thermal treatment, resulting in a considerable increase of the specific surface area of the filters going from 2 to 25.5 m2/g after coating. The virus removal rate was also significantly increased, most notably achieving a 7-log removal, equivalent to 99.99% of the MS2 bacteriophages. Thus, zirconium oxide coating was suggested as promising for virus filtration techniques with potential applications in both water and air treatments.
On the other hand, the removal of positively charged viruses through negatively charged surfaces has been investigated by Michen et al. [25]. Three bacteriophages, Enterobacteria phage MS2 (isoelectric point (IEP) of 3.5, diameter 25 nm), Enterobacteria phage PhiX174 (IEP 6.6, diameter 26 nm) and wild-type bacteriophage Siphovirida (IEP 2.7, diameter 60 nm), were used as model viruses. The results have been rationalized through the use of the DLVO (Derjaguin − Landau − Verwey − Overbeek) theory [25]. Most notably, it was shown that diatomaceous-based ceramic filter, which is widely used to purify water, was not suitable for virus removal due to repulsive forces between viruses and the filter surface besides steric clashes caused by phage specific knobs.
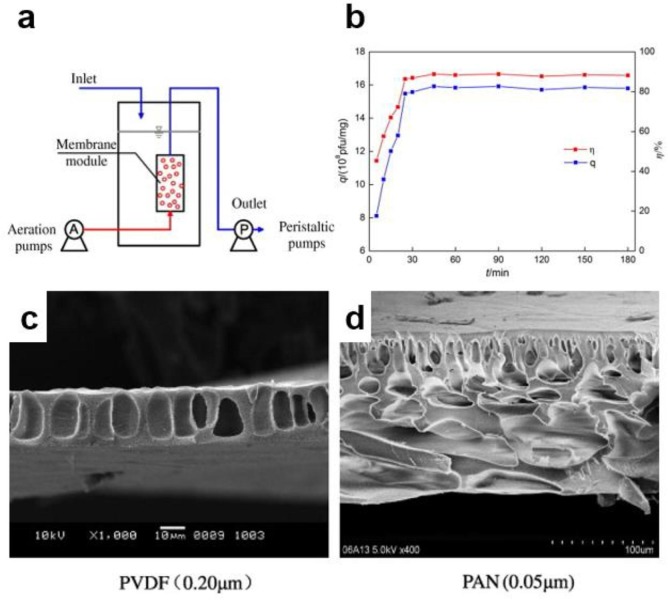
Zheng et al. [57] reported the application of nano-TiO2-membranes for phage f2 removal from water, see Fig. 9 a and 9b. This study showed that the removal of phage f2 was effective after 60 min and the results have been rationalized with the Freundlich adsorption model yielding a qe = 27.4.Ce 1.24. PolyVinyliDene Fluoride (PVDF) (0.20 μm) and PolyAcryloNitrile (PAN) (0.05 μm) membranes were used for TiO2 coupling in order to use nano-TiO2 membranes in flow reactors for water treatment (Fig. 9c and 9d). The experiments demonstrated higher virus removal by PAN (3.88-log) compared to PVDF membrane (6.40-log). The mechanism of virus removal was explained by the electrostatic attraction between the opposite charged nano-TiO2 and phage f2 under acid conditions. The coupled TiO2/membrane system was stable and can be used for virus removal from contaminated water. Silica-decorated TiO2 NPs have been used by Liga et al. [76] for the bacteriophage MS2 inactivation in water. It was shown that doped-TiO2 including 5% of SiO2 has a 37-fold enhanced viral removal performance compared to pristine TiO2. The authors suggested that this removal approach has the advantage of using low-cost and green materials and, hence, is particularly appealing for water treatment.
Fig. 9.
(a) nano-TiO2 membrane adsorption reactor; (b) adsorption kinetics of nano-TiO2 regarding phage F2. SEM images of (c) PVDF (PolyVinyliDene Fluoride) and (d) PAN (PolyAcryloNitrile) flat membranes. Reprinted from [57].
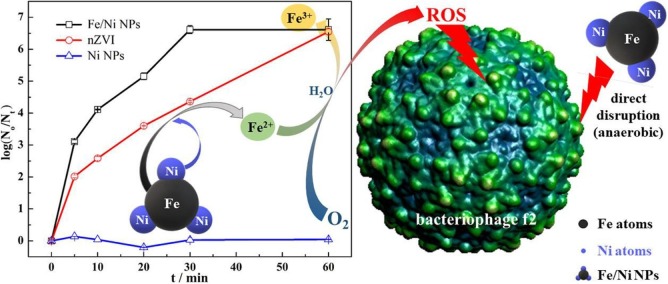
Cheng et al. [59] reported the removal of bacteriophage f2 from water by spherical Fe/Ni NPs (93 nm) under aerobic conditions, see Fig. 10 . Fe/Ni NPs were synthesized by a liquid-phase method using NaBH4 solution. It was found that bacteriophages f2 were completely removed by Fe/Ni NPs after 30 min treatment from a solution with an initial concentration of 4 × 106 PFU/mL (PFU: plaque-forming unit). The effect of the Fe:Ni ratio on virus removal was investigated and results showed that the highest removal efficiency was obtained with a 5:1 ratio. Oxygen condition, pH, initial virus concentration, adsorbent mass and temperature were also tested and analyzed to determine their impacts on the removal of this virus. The inactivation mechanism of bacteriophage f2 was explained by the oxidative stress produced by iron and by the production of reactive oxygen species (OH• and O2 •−) formed due to the oxidation of Fe0 and catalyzed by Ni0 [59].
Fig. 10.
Removal of bacteriophage f2 in water by Fe/Ni nanoparticles. Reprinted from [59].
5. Zeolites
Zeolites are crystalline and porous materials with regular framework structures composed of cages and pores of various size and shape [77]. Zeolites have exceptional chemical selectivity, high adsorption capacity and are biocompatible and safe, consequently, they have attracted considerable interest for biomedical applications. It is now possible to synthesize zeolites with nanosized dimensions and stabilized in colloidal suspensions [78], [79]. The high stability of nanozeolites with regular micropores makes them suitable for selectively adsorbing and desorbing different molecules based on their size allowing them to act as molecular sieves [80]. Furthermore, the tunable chemical composition of these crystalline materials allows them to adapt to different chemical environments and thus to exhibit high stability in acidic and alkaline media [81]. The interest of zeolites as antiviral adsorption agents also resides in the fact that their properties can easily be tuned by the introduction of different metal cations in nanosized materials, most notably altering their adsorption capacity [80], [82], [83], [84], [85]. Although this has mainly be exploited for gas separation, its interest for purification stands out as well. Zeolites can also be specifically functionalized, tuning their surface and interparticle porosity, in order to target a specific tissue or cell type. As an example, antibody-nanozeolite bioconjugates have been generated and successfully employed to target cancer cells [86]. As an extension, and because of their favorable physico-chemical properties zeolites can be efficiently used to remove viruses, also thanks to their high porosity and surface area (see Table 3 ).
Table 3.
Summary of some advantages and limitations of different alternative adsorbents used for virus inactivation.
| Adsorbents | Type of virus | Consequences and advantages |
|---|---|---|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Bright et al. [60] used zeolite as an adsorbent to remove coronavirus 229E from water. They demonstrated that silver- and copper-doped zeolites can induce a significant reduction of the presence of the coronavirus in water solution after 1 h treatment. Indeed, the functionalization of zeolite via silver and copper, i.e. metals presenting known antibacterial properties, has been shown to be an efficient way to inhibit SARS coronavirus, as well as other coronaviruses and human norovirus (calicivirus) [60]. Even if experiences are still scarcer than for other materials, the promising results show that metal-functionalized zeolites can be considered as an excellent, and highly flexible, agent to reduce the contamination by all types of viruses from both water and air.
6. Silica nanoparticles
Silica is a well-known adsorbent that has unique physicochemical properties: large surface area, chemical purity, hydrophilicity, and significant adsorption capacity. Silica has the ability to actively adsorb bioactive molecules, proteins, microorganisms, and viruses [36], [87], [88], [89]. The specific surface area of silica used for medical purposes is 300 m2/g, and is usually characterized as a white powder consisting of spherical particles with a diameter of 9–10 nm. The surface of silica particles contains silanol and hydroxyl groups, which are interacting via hydrogen bonds [90]. In addition, water molecules are present at the silica surface both chemically bonded or physically adsorbed. The concentration of silanol groups, which are the main adsorption sites, is approximately 0.6–0.7 mmol/g or 2–2.5 μmol/m2 [91], [92].
Silica has a significant protein adsorption capacity, for example, 1 g of silica can adsorb up to 200–300 mg/g of gelatin or 800 mg/g of albumin from aqueous solution at pH 5–6. Silica is an active adsorbent of various kind of microorganisms: indeed 108 up to 1010 microbial bodies can be adsorbed per 1 g of silica. The properties and structure of the active centers of the SiO2 surface, which determine the specific interaction with the biological objects, are described in [93], [94], [95], [96].
As such silica-based NPs are particularly attractive for virus removal as highlighted in Table 3. Various methods of SiO2 surface modification have been described in order to increase its adsorption selectivity towards microorganisms [97], [98], [99], [100], [101]. Chemically modified silicas are of particular interest among nanoporous mineral oxides [102]. They have a developed surface and are not only excellent adsorbents in their original form, but they are also capable of radically changing their physicochemical properties as a result of chemical modification [103]. A variety of NH2-containing moieties can be used to modify silica, for example, lupamin, 3–3-(ethylenediamino) propyl, aminopropyl, 3-(diethylenetriamino) propyl [104]. Such modified functionalized silica particles show high efficiency towards bacteriophage MS-2 removal (greater than98%). The mechanism of virus inactivation is explained by the high density of positively charged NH2-groups, electrostatic interaction and hydrogen bonds formation.
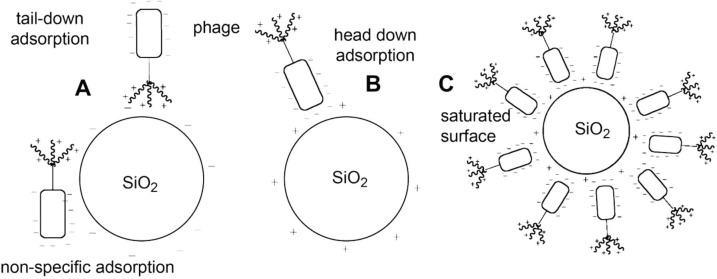
As another example, magnetic silicon microspheres have been used for detection of Japanese encephalitis virus [105]. Novel amine-functionalized magnetic Fe3O4–SiO2–NH2 NPs have been investigated as adsorbent for bacteriophage f2 and Poliovirus-1 removal by Zhan et al. [106]. NPs were obtained through layer-by-layer method using Fe(acac)3, tetraethyl orthosilicate (TEOS) and γ-amino propyltriethoxy-silane (APTES) as precursors. The adsorption mechanism was rationalized by the surface charge, hydrophobicity and surface properties of pathogens and NPs matrix. The electrostatic attraction between the negatively charged Poliovirus-1 walls and the positively charged adsorbent surface was clearly identified, and it was shown that the removal efficiency of Fe3O4–SiO2–NH2 NPs over bacteriophage f2 and Poliovirus-1 exceeded 97%. Cademartiri et al. [107] have explored an alternative strategy in which four unmodified phages were immobilized on anionic silica substrate via electrostatically-facilitated physisorption, see Fig. 11 . The proposed adsorbents can be used to detect and control pathogens, particularly drug resistant bacteria.
Fig. 11.
Electrostatic attraction model between phage and charged SiO2 particles. A: tail down and non-specific adsorption, B: head down, C: head down saturated surface [107].
7. Metal–Organic frameworks (MOFs)
MOFs are composed of inorganic nodes of metallic species/clusters and organic ligands. These materials are very stable under different operating conditions (e.g., temperature and solvents) [108]. Several studies have concluded that they offer a spectrum of possibilities to prepare new materials including biocomposites [108], [109]. The application of MOFs-based biocomposites covers biocatalysis, DNA detection, sensing, drug delivery and immobilization [109]. It has been shown that the textural properties (pore size and volume), crystal morphology and chemical functionality of MOFs can be modulated to obtain specific performances for a variety of applications [110], [111]. MOFs properties can be tailored to encapsulate proteins and other biological molecules preserving their functionalities [109]. These materials are also capable of encapsulating bacteria and viruses in which case adsorption plays a fundamental role [108]. These findings open a broad spectrum of possibilities to employ MOFs for facing the environmental and public health challenges caused by viruses, see Table 3.
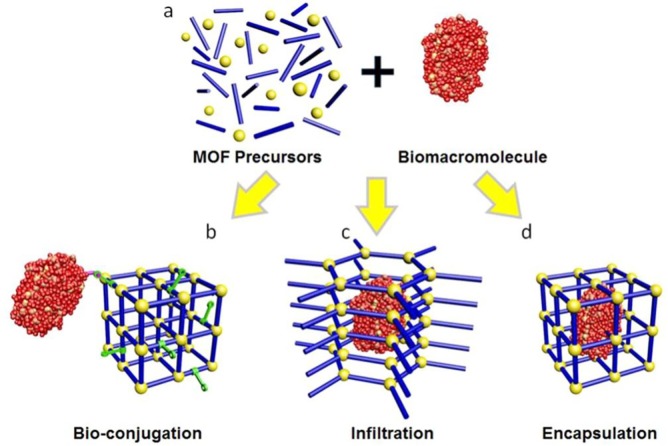
The analysis and study of biological-inorganic matrixes obtained from MOFs are relevant topics to improve and enhance the environmental and medical applications of this new type of biomaterials. The organic functionalities of MOFs play an important role in the anchorage of biomacromolecules for biocomposites synthesis. For instance, Doonan et al. [109] discussed and analyzed the mechanisms of the incorporation of biological compounds on MOFs, which can be performed via encapsulation, infiltration or bioconjugation (Fig. 12 ). Note that MOFs structures can host a wide variety of both inorganic and organic compounds including biological macromolecules. Parameters like the composition, topology, porosity and surface functionalities of MOFs are important and should be analyzed during the synthesis of biocomposites and to perform the coating of living systems (i.e., biomimetic mineralization) [108].
Fig. 12.
Mechanisms for the incorporation of biological compounds on MOFs structures. Reprinted from [108].
However, it has been recognized that the incorporation of biomacromolecules on the MOFs pore structures implies several technological challenges. Previous studies have indicated that biomacromolecules encapsulated on MOFs can be released by changes in the medium conditions, for example, pH [109]. On the other hand, MOFs can be used in vaccine developments based on the fact that these materials can coat and work as exoskeletons to protect living cells from aggressive environments [112]. Ricco et al. [108] have concluded that MOF can be used to improve the cold chain with the aim of avoiding the degradation of drugs and vaccine. It has been also suggested that hybrid biosynthetic materials can be prepared from MOFs to improve the pharmacokinetics of viral nanoparticles thus affecting the immune response [108]. Vaccine design based on MOFs is a promising area that however still needs to be much largely explored and studied to reach commercial applications.
Finally, MOFs-based materials have also been used to develop sensor for detecting Zika [113], [114], Dengue [113], human immune deficiency virus-1 (HIV-1) [115] and Japanese encephalitis virus [116] with promising results.
8. Clay and clay minerals
A variety of promising adsorbents obtained from clays and their composites has also been proposed to remove rotaviruses and coronaviruses [105], see Table 3. Indeed, the diameter of rotavirus and coronavirus particles are 60–80 and 60–220 nm [117], respectively, which is clearly larger than the size of pores of different adsorbent particles. Hence, both viruses can be adsorbed on the outer surface of adsorbents. The experimental results demonstrated that clay-based adsorbents showed good (70–90%) to excellent (greater than90%) capability to remove bovine rotavirus and bovine coronavirus [117]. Based on this investigation, it was suggested that the involved interactions between the adsorbent surfaces and viruses is probably due to a non-specific protein binding.
9. Viruses adsorption by carbohydrates and their derivatives
Several biological molecules, carbohydrates in particular, have shown effective antiviral activities; these materials are also summarized in Table 3. Such activity is usually due to the competition with the host cell for the interaction with viral capsid proteins, hence preventing the first step of cell infections. Some of the most efficient agents in this context mimic the sugars present at the cell surface, in particular heparan-sulfate proteoglycans that are responsible for the initial viral attachment [118], [119], [120]. The chemical structure of these carbohydrates, their origins and their antiviral properties are briefly described below.
9.1. Antiviral effect of sulfated polysaccharides
Various polysaccharides from plants, marine vegetables, algae and lichens have received increasing attention due to their ability to scavenge free radicals, to activate immune systems, to inhibit lipid peroxidation and to inhibit viral replication [121]. Consequently, these polysaccharides have exhibited numerous biological activities including anticoagulant, antiviral, antioxidative, anti-inflammatory, antiangiogenic, antithrombotic and anticancer effects [122], [123], [124], [125], [126], [127]. Sulfated polysaccharides (SPs) especially from sea algae such as fucoidans, carrageenans, heparin, neem, laminarans and ulvansare are considered as the most interesting and attractive carbohydrates showing antiviral properties [128], [129], [130], [131], [132], [133].
Since 1978, Richards and coworkers [134] have recognized the inhibition of viruses by polysaccharide fractions from marine algae. Recently, other studies have reported that several plants and marine algae contain significant quantities of complex sulfated polysaccharides that can exhibit antiviral activity against serious infectious diseases such as HIV [135], yellow fever virus [136], dengue virus type 1 [136], dengue virus type 2 [137] and herpes simplex viruses [133], [138], [139], [140].
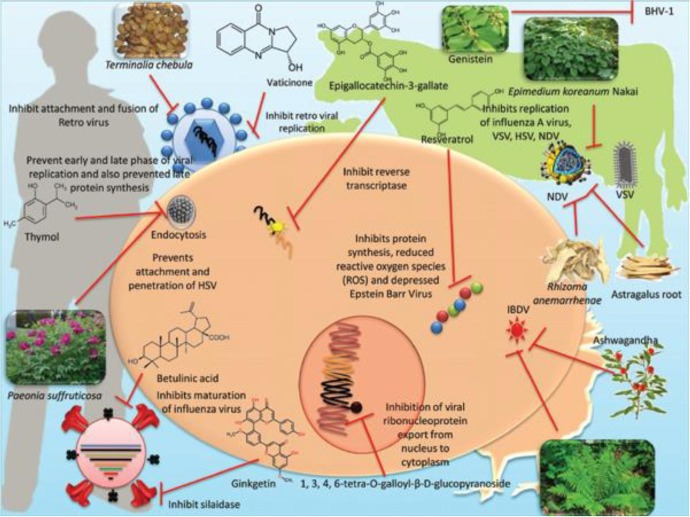
A number of hypotheses has been proposed to explain the antiviral mechanisms of sulfated polysaccharides (see Fig. 13 ) including direct interaction with the viral particle, alteration of viruses’ adsorption or attachment to cell receptors, inhibition of the penetration of virus into the host cell, the interference with different stages of viral replication [141], [142], [143] .
Fig. 13.
Antiviral action mechanisms of medicinal plant metabolites. Reprinted from [143].
On the other hand, numerous studies have reported efficient strategies, based on synthetic pathways, enzymatic digestion, partial hydrolysis and methylation methods, to produce sulfated derivatives of different polysaccharides such as dextran, pentosan, dermatan, carrageenan, alginate, xylomannan and galactan [123], [135], [144]. Furthermore, it has been highlighted that the presence of sulfated groups enhanced the antiviral properties, and is hence essential for viruses’ inactivation. More specifically, it has been noted that while the virucidal efficacy can increase with the degree of sulfation and the specific position of the sulfate ester groups, it also depends on the molecular weight, the constituent sugars, their conformation and dynamic stereochemistry [132], [145], [146]. Generally, the results have indicated that the greater the degree of sulfation, the higher the biological activity, an observation which could conduct to find a drug candidate with higher potency and less cytotoxicity [141], [147], [148], since polysaccharides with low sulfation degrees are in general inactive against viruses [145]. Some applications of these natural compounds in virus inactivation have already been reported [121], [137]. For example, Hidari et al. [137] have found that dengue virus particles bounded exclusively to fucoidan via their envelope glycoprotein thus indicating that sulfated polysaccharides could be developed as a potential inhibitory agent. Kim et al. [121] reported that marine sulfated polysaccharides presented numerous advantages such as relatively low production costs, broad spectrum of antiviral properties, low cytotoxicity, safety, wide acceptability and novel modes of action over other classes of antiviral drugs. Hence, they suggested the use of these sulfated polysaccharides as promising decontamination agents in the near future.
9.2. Antiviral action of modified and sulfated cyclodextrins
Cyclodextrins (CDs) are natural cyclic oligosaccharides formed by α-(1–4)-linked glucose units [149], [150]. The most common CDs, referred as α-, β- and γ-CDs, have 6, 7 and 8 glucopyranoside moieities, respectively. These cyclic natural compounds have attracted a considerable attention especially for their biological applications due to their ability to encapsulate different compounds being, consequently, used as molecular carriers [151]. Thus, CDs have found numerous applications in commercial and industrial fields including drug delivery [151], [152], gene delivery [153], bioimaging [154], photodynamic therapy [153], air fresheners [153], cosmetics [153], food processing [155] and environmental depollution [156], [157]. As molecular carriers, CDs have been employed to enhance the antiviral activity of a phosphodiester oligodeoxynucleotide [158]. This study showed that an important increase of the antiviral activity (90% inhibition) was obtained with only 7.5 mM oligonucleotide complexed to a cyclodextrin derivative, the 6-deoxy-6-S-b-D-galactopyranosyl-6-thio-cyclomaltoheptaose, in the 1:100 ratio. The authors suggested that the use of cyclodextrin derivatives as carrier for phosphodiester oligonucleotides delivery could be an effective method for increasing the therapeutic potential of these compounds against viral infections. On the other hand, De Clercq [159] reported that the modified cyclodextrin sulfates acted, by themselves, as anti-HIV agents since they interfered with the adsorption stage of HIV replicative cycle. It was also concluded that the presence of sulfate groups was necessary to induce the anti-HIV activity as it is essential for the inhibition of the virus-cell binding. CDs chemical modifications, like those reported for the modified β-cyclodextrin sulfates (mCDS71 and mCDS11), is a promising approach to achieve high oral bioavailability and absorption [160], [161], [162]. Further studies have also confirmed the antiviral properties of sulfonated CDs against HIV [163], [164]. However, their action was found to be virus specific and reversible. Recently, Jones et al. [120] have prepared cyclodextrins with mercaptoundecane sulfonic acids, to mimic heparan sulfates and obtained outstanding anti-viral properties. They showed that the modified CDs, while being nontoxic and biocompatible, possessed in vitro broad-spectrum virucidal properties at micromolar concentrations against several viruses including herpes simplex virus (HSV), respiratory syncytial virus (RSV), dengue virus, and Zika virus. Their results also indicate that modified CDs were effective ex vivo against both laboratory and clinical strains of RSV and HSV-2 in respiratory and vaginal tissue culture models, respectively. Additionally, they were also effective when administrated in mice before intra vaginal HSV-2 inoculation. Finally, these modified CDs were successful in overcoming a mutation resistance test that the available anti-HSV drug (acyclovir) failed.
10. Conclusion and perspectives
This review covers the potential application of a number of adsorbents or inhibitors for the treatment of water and air polluted by viruses and to the development of potential antivirals treatment. Overall, the viruses’ properties and classification have been discussed, including the available adsorbents that can be used for their removal or inactivation. Indeed, while a variety of adsorbents can be used to remove different viruses present in the environment, their performance is affected by both the structural parameters and their surface chemistry. It is clear from the literature review that the surface charge of adsorbents is a paramount parameter for the effective viral inactivation in both air and water, that can be tailored via chemical functionalization [165], [166]. Indeed, the electrostatic potential of adsorbent surface depends on the concentration and type of functional groups that are exposed at the adsorbent/fluid interface [166]. The adsorbent surface composition can be modified via the incorporation of a variety of doping elements thus improving the surface interactions with viruses. Note that the identification of the best chemical functionalization protocol to enhance the surface properties of a specific adsorbent and, consequently, its corresponding antiviral activity is paramount to reduce the purification costs. Also, the analysis of surface potential and antiviral properties of different adsorbents is a relevant topic to be explored in forthcoming studies.
Globally, it appears that nanoparticles have a tremendous potential to develop effective and low-cost sanitation systems for air and water purification. On the other hand, biological compounds like polysaccharides and cyclodextrins may represent interesting alternative to develop novel adsorbents for the removal of viruses from fluids. The production or extraction of these chemical compounds, as well as their inclusion in everyday life objects (e.g., plastic surfaces, textiles, hand gel) represents a most important challenge that the scientific community has to undertake to fight against important public health threats.
As stated in this review, practical methods applied for virus removal include membrane filtration, chemical disinfection with chlorine or ozone and ultraviolet disinfection [27], [35]. However, these methods may face different economical and/or technical limitations. For instance, the application of chemicals for water disinfection (e.g., chlorination and ozonation) has been reduced in some countries due to the generation of toxic byproducts (e.g., haloacetic acids, trihalomethane) from the degradation or transformation of the disinfectants [27], [167]. Membrane technologies are effective for virus removal [27] but their main drawback is associated with their operating costs, which can be very high and hence prohibitive for large scale use in underdeveloped countries [11]. Previous studies have also reported that some viruses (e.g., Norovirus) can resist the UV radiation thus limiting the efficacy of this process [168]. Adsorbents already available in the market for water disinfection include traditional filters with activated carbons doped with silver [169], chemically modified polymers as ion exchange resins [170] and even MOFs [171]. Overall, available commercial adsorbents may lack an effective antiviral activity especially for new viruses besides relatively high production costs that are mainly due to the specific compound used to confer antiviral properties of the adsorbent surface. Indeed, in some cases they comprise high-added values compound which can be used for other technological applications thus increasing their demand and, consequently, their market price.
Specific emphasis should then be placed on the development of efficient and low-cost purification processes that will allow the large-scale treatment of fluids in developing or underdeveloped countries to avoid the uncontrolled diffusion, or mutation, of dangerous viral agents resulting in global threats. The production of self-sanitizing objects will be particularly attractive and will require a considerable effort from the material science community in the production of efficient, flexible, and miniaturized purifying agents. It is clear, as also shown by the COVID-19 pandemic, that developing novel adsorbents with outstanding antiviral properties will be a permanent and continuous necessity to face the presence of known and unknown viruses in the environment.
CRediT authorship contribution statement
Lotfi Sellaoui: Conceptualization, Methodology, Data curation, Writing - original draft, Visualization, Investigation, Writing - review & editing. Michael Badawi: Conceptualization, Methodology, Data curation, Writing - original draft, Visualization, Investigation, Writing - review & editing. Antonio Monari: Data curation, Writing - original draft, Writing - review & editing. Tetiana Tatarchuk: Conceptualization, Methodology, Data curation, Writing - original draft, Visualization, Investigation. Sonia Jemli: Data curation, Writing - original draft, Visualization, Investigation. Guilherme Luiz Dotto: Data curation, Writing - original draft, Visualization, Investigation. Adrian Bonilla-Petriciolet: Data curation, Writing - original draft, Visualization, Investigation, Writing - review & editing. Zhuqi Chen: Visualization, Investigation, Supervision, Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was financially supported by the National Key R&D Program of China (No. 2018YFC1901403), the National Science Foundation of China (No. 21671072), the Fundamental Research Funds for the Central Universities (No. 2019kfyRCPY058), and Chutian Scholar Foundation from Hubei province.
References
- 1.Zhang N., Wang L., Deng X., Liang R., Su M., He C., Hu L., Su Y., Ren J., Yu F., Du L., Jiang S. Recent advances in the detection of respiratory virus infection in humans. J. Med. Virol. 2020;92(4):408–417. doi: 10.1002/jmv.v92.410.1002/jmv.25674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.J. Guarner Three Emerging Coronaviruses in Two Decades 153 4 2020 2020 420 421 10.1093/ajcp/aqaa029. [DOI] [PMC free article] [PubMed]
- 3.W.-S. Ryu, Molecular Virology of Human Pathogenic Viruses, Elsevier Inc., 2017.
- 4.Artika I.M., Wiyatno A., Ma'roef C.N. Pathogenic viruses: Molecular detection and characterization. Infect. Genet. Evol. 2020;81:104215. doi: 10.1016/j.meegid.2020.104215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elkholy A.A., Grant R., Assiri A., Elhakim M., Malik M.R., Van Kerkhove M.D. MERS-CoV infection among healthcare workers and risk factors for death: Retrospective analysis of all laboratory-confirmed cases reported to WHO from, to 2 June 2018. J. Infect. Public Health. 2012;13(2019):418–422. doi: 10.1016/j.jiph.2019.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R., Niu P., Zhan F., Ma X., Wang D., Xu W., Wu G., Gao G.F., Tan W. A novel coronavirus from patients with pneumonia in China. N. Engl. J. Med. 2019;382(2020):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sohrabi C., Alsafi Z., O'Neill N., Khan M., Kerwan A., Al-Jabir A., Iosifidis C., Agha R. novel coronavirus (COVID-19) Int. J. Surg. 2020;76:71–76. doi: 10.1016/j.ijsu.2020.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Domingo José.L., Marquès M., Rovira J. Influence of airborne transmission of SARS-CoV-2 on COVID-19 pandemic. A review. Environ. Res. 2020;188:109861. doi: 10.1016/j.envres.2020.109861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mohan S.V., Hemalatha M., Kopperi H., Ranjith I., Kumar A.K. SARS-CoV-2 in environmental perspective: Occurrence, persistence, surveillance, inactivation and challenges. Chem. Eng. J. 2021;405:126893. doi: 10.1016/j.cej.2020.126893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Medema G., Heijnen L., Elsinga G., Italiaander R., Brouwer A. Presence of SARS-Coronavirus-2 RNA in Sewage and Correlation with Reported COVID-19 Prevalence in the Early Stage of the Epidemic in the Netherlands. Environ. Sci. Technol. Lett. 2020;7(7):511–516. doi: 10.1021/acs.estlett.0c0035710.1021/acs.estlett.0c00357.s001. [DOI] [PubMed] [Google Scholar]
- 11.Adelodun B., Ajibade F.O., Ighalo J.O., Odey G., Ibrahim R.G., Kareem K.Y., Bakare H.O., Tiamiyu A.O., Ajibade T.F., Abdulkadir T.S., Adeniran K.A., Choi K.S. Assessment of socioeconomic inequality based on virus-contaminated water usage in developing countries: A review. Environ. Res. 2021;192 doi: 10.1016/j.envres.2020.110309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lahrich S., Laghrib F., Farahi A., Bakasse M., Saqrane S., El Mhammedi M.A. Review on the contamination of wastewater by COVID-19 virus: Impact and treatment. Sci. Total Environ. 2021;751 doi: 10.1016/j.scitotenv.2020.142325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pisharody L., Suresh S., Mukherji S. Evaluation of adsorbents and eluents for application in virus concentration and adsorption-desorption isotherms for coliphages. Chem. Eng. J. 2021;403 doi: 10.1016/j.cej.2020.126267. [DOI] [Google Scholar]
- 14.Gerba C.P., Betancourt W.Q., Kitajima M. How much reduction of virus is needed for recycled water: A continuous changing need for assessment? Water Res. 2017;108:25–31. doi: 10.1016/j.watres.2016.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carducci A., Federigi I., Liu D., Thompson J.R., Verani M. Making Waves: Coronavirus detection, presence and persistence in the water environment: State of the art and knowledge needs for public health. Water Res. 2020;179 doi: 10.1016/j.watres.2020.115907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park D.H., Joe Y.H., Piri A., An S., Hwang J. Determination of Air Filter Anti-Viral Efficiency against an Airborne Infectious Virus. J. Hazard. Mater. 2020;396 doi: 10.1016/j.jhazmat.2020.122640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haramoto E., Kitajima M., Hata A., Torrey J.R., Masago Y., Sano D., Katayama H. A review on recent progress in the detection methods and prevalence of human enteric viruses in water. Water Res. 2018;135:168–186. doi: 10.1016/j.watres.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 18.Morrison C.M., Betancourt W.Q., Quintanar D.R., Lopez G.U., Pepper I.L., Gerba C.P. Potential indicators of virus transport and removal during soil aquifer treatment of treated wastewater effluent. Water Res. 2020;177 doi: 10.1016/j.watres.2020.115812. [DOI] [PubMed] [Google Scholar]
- 19.Gerba C.P., Betancourt W.Q., Kitajima M., Rock C.M. Reducing uncertainty in estimating virus reduction by advanced water treatment processes. Water Res. 2018;133:282–288. doi: 10.1016/j.watres.2018.01.044. [DOI] [PubMed] [Google Scholar]
- 20.Springthorpe S., Sattar S.A. Chapter 6 Virus Removal During Drinking Water Treatment. Perspect. Med. Virol. 2007;17:109–126. doi: 10.1016/S0168-7069(07)17006-3. [DOI] [Google Scholar]
- 21.Nilsen V., Christensen E., Myrmel M., Heistad A. Spatio-temporal dynamics of virus and bacteria removal in dual-media contact-filtration for drinking water. Water Res. 2019;156:9–22. doi: 10.1016/j.watres.2019.02.029. [DOI] [PubMed] [Google Scholar]
- 22.Delanka-Pedige H.M.K., Munasinghe-Arachchige S.P., Zhang Y., Nirmalakhandan N. Bacteria and virus reduction in secondary treatment: Potential for minimizing post disinfectant demand. Water Res. 2020;177 doi: 10.1016/j.watres.2020.115802. [DOI] [PubMed] [Google Scholar]
- 23.Casanova L., Rutala W.A., Weber D.J., Sobsey M.D. Survival of surrogate coronaviruses in water. Water Res. 2009;43:1893–1898. doi: 10.1016/j.watres.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heider S., Metzner C. Quantitative real-time single particle analysis of virions. Virology. 2014;462–463:199–206. doi: 10.1016/j.virol.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Michen B., Meder F., Rust A., Fritsch J., Aneziris C., Graule T. Virus removal in ceramic depth filters based on diatomaceous earth. Environ. Sci. Technol. 2012;46(2):1170–1177. doi: 10.1021/es2030565. [DOI] [PubMed] [Google Scholar]
- 26.Michen B., Fritsch J., Aneziris C., Graule T. Improved virus removal in ceramic depth filters modified with MgO. Environ. Sci. Technol. 2013;47:1526–1533. doi: 10.1021/es303685a. [DOI] [PubMed] [Google Scholar]
- 27.Goswami K.P., Pugazhenthi G. Credibility of polymeric and ceramic membrane filtration in the removal of bacteria and virus from water: A review. J. Environ. Manage. 2020;268:110583. doi: 10.1016/j.jenvman.2020.110583. [DOI] [PubMed] [Google Scholar]
- 28.Shirasaki N., Matsushita T., Matsui Y., Murai K., Aochi A. Elimination of representative contaminant candidate list viruses, coxsackievirus, echovirus, hepatitis A virus, and norovirus, from water by coagulation processes. J. Hazard. Mater. 2017;326:110–119. doi: 10.1016/j.jhazmat.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 29.Betancourt W.Q., Schijven J., Regnery J., Wing A., Morrison C.M., Drewes Jörg.E., Gerba C.P. Variable non-linear removal of viruses during transport through a saturated soil column. J. Contam. Hydrol. 2019;223:103479. doi: 10.1016/j.jconhyd.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 30.Prado T., de Castro Bruni A., Barbosa M.R.F., Garcia S.C., de Jesus Melo A.M., Sato M.I.Z. Performance of wastewater reclamation systems in enteric virus removal. Sci. Total Environ. 2019;678:33–42. doi: 10.1016/j.scitotenv.2019.04.435. [DOI] [PubMed] [Google Scholar]
- 31.Sidhu J.P.S., Sena K., Hodgers L., Palmer A., Toze S. Comparative enteric viruses and coliphage removal during wastewater treatment processes in a sub-tropical environment. Sci. Total Environ. 2018;616–617:669–677. doi: 10.1016/j.scitotenv.2017.10.265. [DOI] [PubMed] [Google Scholar]
- 32.Randazzo W., Truchado P., Cuevas-Ferrando E., Simón P., Allende A., Sánchez G. SARS-CoV-2 RNA in wastewater anticipated COVID-19 occurrence in a low prevalence area. Water Res. 2020;181 doi: 10.1016/j.watres.2020.115942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miura T., Okabe S., Nakahara Y., Sano D. Removal properties of human enteric viruses in a pilot-scale membrane bioreactor (MBR) process. Water Res. 2015;75:282–291. doi: 10.1016/j.watres.2015.02.046. [DOI] [PubMed] [Google Scholar]
- 34.Zhang C., Li Y., Shuai D., Shen Y., Wang D. Progress and challenges in photocatalytic disinfection of waterborne Viruses: A review to fill current knowledge gaps. Chem. Eng. J. 2019;355:399–415. doi: 10.1016/j.cej.2018.08.158. [DOI] [Google Scholar]
- 35.Habibi-Yangjeh A., Asadzadeh-Khaneghah S., Feizpoor S., Rouhi A. Review on heterogeneous photocatalytic disinfection of waterborne, airborne, and foodborne viruses: Can we win against pathogenic viruses? J. Colloid Interface Sci. 2020;580:503–514. doi: 10.1016/j.jcis.2020.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.La Rosa G., Bonadonna L., Lucentini L., Kenmoe S., Suffredini E. Coronavirus in water environments: Occurrence, persistence and concentration methods - A scoping review. Water Res. 2020;179 doi: 10.1016/j.watres.2020.115899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cheek E., Guercio V., Shrubsole C., Dimitroulopoulou S. Portable air purification: Review of impacts on indoor air quality and health. Sci. Total Environ. 2020:142585. doi: 10.1016/j.scitotenv.2020.142585. [DOI] [PubMed] [Google Scholar]
- 38.Wu B., Wang R., Fane A.G. The roles of bacteriophages in membrane-based water and wastewater treatment processes: A review. Water Res. 2017;110:120–132. doi: 10.1016/j.watres.2016.12.004. [DOI] [PubMed] [Google Scholar]
- 39.Carvajal G., Branch A., Sisson S.A., Roser D.J., van den Akker B., Monis P., Reeve P., Keegan A., Regel R., Khan S.J. Virus removal by ultrafiltration: Understanding long-term performance change by application of Bayesian analysis. Water Res. 2017;122:269–279. doi: 10.1016/j.watres.2017.05.057. [DOI] [PubMed] [Google Scholar]
- 40.Lu R., Zhang C., Piatkovsky M., Ulbricht M., Herzberg M., Nguyen T.H. Improvement of virus removal using ultrafiltration membranes modified with grafted zwitterionic polymer hydrogels. Water Res. 2017;116:86–94. doi: 10.1016/j.watres.2017.03.023. [DOI] [PubMed] [Google Scholar]
- 41.Junter G.-A., Lebrun L. Cellulose-based virus-retentive filters: a review. Rev. Environ. Sci. Biotechnol. 2017;16(3):455–489. doi: 10.1007/s11157-017-9434-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wegmann M., Michen B., Luxbacher T., Fritsch J., Graule T. Modification of ceramic microfilters with colloidal zirconia to promote the adsorption of viruses from water. Water Res. 2008;42(6-7):1726–1734. doi: 10.1016/j.watres.2007.10.030. [DOI] [PubMed] [Google Scholar]
- 43.Bofill-Mas Sílvia, Rusiñol M. Recent trends on methods for the concentration of viruses from water samples. Curr. Opin. Environ. Sci. Heal. 2020;16:7–13. doi: 10.1016/j.coesh.2020.01.006. [DOI] [Google Scholar]
- 44.Manukyan L., Padova J., Mihranyan A. Virus removal filtration of chemically defined Chinese Hamster Ovary cells medium with nanocellulose-based size exclusion filter. Biologicals. 2019;59:62–67. doi: 10.1016/j.biologicals.2019.03.001. [DOI] [PubMed] [Google Scholar]
- 45.Rambags F., Tanner C.C., Stott R., Schipper L.A. Bacteria and virus removal in denitrifying bioreactors: Effects of media type and age. Ecol. Eng. 2019;138:46–53. doi: 10.1016/j.ecoleng.2019.06.021. [DOI] [Google Scholar]
- 46.Shimabuku Q.L., Ueda-Nakamura T., Bergamasco R., Fagundes-Klen M.R. Chick-Watson kinetics of virus inactivation with granular activated carbon modified with silver nanoparticles and/or copper oxide. Process Saf. Environ. Prot. 2018;117:33–42. doi: 10.1016/j.psep.2018.04.005. [DOI] [Google Scholar]
- 47.Matsushita T., Suzuki H., Shirasaki N., Matsui Y., Ohno K. Adsorptive virus removal with super-powdered activated carbon. Sep. Purif. Technol. 2013;107:79–84. doi: 10.1016/j.seppur.2013.01.017. [DOI] [Google Scholar]
- 48.Powell T., Brion G.M., Jagtoyen M., Derbyshire F. Investigating the effect of carbon shape on virus adsorption. Environ. Sci. Technol. 2000;34(13):2779–2783. doi: 10.1021/es991097w. [DOI] [Google Scholar]
- 49.Shimabuku Q.Letícia., Arakawa Flávia.S., Fernandes Silva M., Ferri Coldebella P., Ueda-Nakamura Tânia, Fagundes-Klen Márcia.R., Bergamasco R. Water treatment with exceptional virus inactivation using activated carbon modified with silver (Ag) and copper oxide (CuO) nanoparticles. Environ. Technol. (United Kingdom). 2017;38(16):2058–2069. doi: 10.1080/09593330.2016.1245361. [DOI] [PubMed] [Google Scholar]
- 50.Junter G.-A., Lebrun L. Polysaccharide-based chromatographic adsorbents for virus purification and viral clearance. J. Pharm. Anal. 2020;10(4):291–312. doi: 10.1016/j.jpha.2020.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xing Y., Ellis A., Magnuson M., Harper W.F. Adsorption of bacteriophage MS2 to colloids: Kinetics and particle interactions. Colloids Surfaces A Physicochem. Eng. Asp. 2020;585:124099. doi: 10.1016/j.colsurfa.2019.124099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Joe Y.H., Park D.H., Hwang J. Evaluation of Ag nanoparticle coated air filter against aerosolized virus: Anti-viral efficiency with dust loading. J. Hazard. Mater. 2016;301:547–553. doi: 10.1016/j.jhazmat.2015.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Minoshima M., Lu Y., Kimura T., Nakano R., Ishiguro H., Kubota Y., Hashimoto K., Sunada K. Comparison of the antiviral effect of solid-state copper and silver compounds. J. Hazard. Mater. 2016;312:1–7. doi: 10.1016/j.jhazmat.2016.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chrysikopoulos C.V., Aravantinou A.F. Virus inactivation in the presence of quartz sand under static and dynamic batch conditions at different temperatures. J. Hazard. Mater. 2012;233–234:148–157. doi: 10.1016/j.jhazmat.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 55.Mi X., Heldt C.L. Adsorption of a non-enveloped mammalian virus to functionalized nanofibers. Colloids Surfaces B Biointerfaces. 2014;121:319–324. doi: 10.1016/j.colsurfb.2014.06.007. [DOI] [PubMed] [Google Scholar]
- 56.Chung J.W., Foppen J.W., Gerner G., Krebs R., Lens P.N.L. Removal of rotavirus and adenovirus from artificial ground water using hydrochar derived from sewage sludge. J. Appl. Microbiol. 2015;119(3):876–884. doi: 10.1111/jam.12863. [DOI] [PubMed] [Google Scholar]
- 57.Zheng X., Chen D., Wang zhiwei, Lei Y., Cheng R. Nano-TiO2 membrane adsorption reactor (MAR) for virus removal in drinking water. Chem. Eng. J. 2013;230:180–187. doi: 10.1016/j.cej.2013.06.069. [DOI] [Google Scholar]
- 58.Tiliket G., Le Sage D., Moules V., Rosa-Calatrava M., Lina B., Valleton J.M., Nguyen Q.T., Lebrun L. A new material for airborne virus filtration. Chem. Eng. J. 2011;173:341–351. doi: 10.1016/j.cej.2011.07.059. [DOI] [Google Scholar]
- 59.Cheng R., Kang M., Zhuang S., Wang S., Zheng X., Pan X., Shi L., Wang J. Removal of bacteriophage f2 in water by Fe/Ni nanoparticles: Optimization of Fe/Ni ratio and influencing factors. Sci. Total Environ. 2019;649:995–1003. doi: 10.1016/j.scitotenv.2018.08.380. [DOI] [PubMed] [Google Scholar]
- 60.Bright K.R., Sicairos-Ruelas E.E., Gundy P.M., Gerba C.P. Assessment of the Antiviral Properties of Zeolites Containing Metal Ions. Food Environ. Virol. 2009;1:37–41. doi: 10.1007/s12560-008-9006-1. [DOI] [Google Scholar]
- 61.Chisholm P.J., Busch J.W., Crowder D.W. Effects of life history and ecology on virus evolutionary potential. Virus Res. 2019;265:1–9. doi: 10.1016/j.virusres.2019.02.018. [DOI] [PubMed] [Google Scholar]
- 62.Zandi R., Dragnea B., Travesset A., Podgornik R. On virus growth and form. Phys. Rep. 2020;847:1–102. doi: 10.1016/j.physrep.2019.12.005. [DOI] [Google Scholar]
- 63.Gamazo P., Victoria M., Schijven J.F., Alvareda E., Tort L.F.L., Ramos J., Lizasoain L.A., Sapriza G., Castells M., Bessone L., Colina R. Modeling the Transport of Human Rotavirus and Norovirus in Standardized and in Natural Soil Matrix-Water Systems. Food Environ. Virol. 2020;12(1):58–67. doi: 10.1007/s12560-019-09414-z. [DOI] [PubMed] [Google Scholar]
- 64.Manukyan L., Li P., Gustafsson S., Mihranyan A. Growth media filtration using nanocellulose-based virus removal filter for upstream biopharmaceutical processing. J. Memb. Sci. 2019;572:464–474. doi: 10.1016/j.memsci.2018.11.002. [DOI] [Google Scholar]
- 65.Bartels J., Batista A.G., Kroll S., Maas M., Rezwan K. Hydrophobic ceramic capillary membranes for versatile virus filtration. J. Memb. Sci. 2019;570–571:85–92. doi: 10.1016/j.memsci.2018.10.022. [DOI] [Google Scholar]
- 66.Armanious A., Aeppli M., Jacak R., Refardt D., Sigstam T., Kohn T., Sander M. Viruses at Solid-Water Interfaces: A Systematic Assessment of Interactions Driving Adsorption. Environ. Sci. Technol. 2016;50(2):732–743. doi: 10.1021/acs.est.5b0464410.1021/acs.est.5b04644.s00110.1021/acs.est.5b04644.s002. [DOI] [PubMed] [Google Scholar]
- 67.Meder F., Wehling J., Fink A., Piel B., Li K., Frank K., Rosenauer A., Treccani L., Koeppen S., Dotzauer A., Rezwan K. The role of surface functionalization of colloidal alumina particles on their controlled interactions with viruses. Biomaterials. 2013;34(17):4203–4213. doi: 10.1016/j.biomaterials.2013.02.059. [DOI] [PubMed] [Google Scholar]
- 68.Heldt C.L., Zahid A., Vijayaragavan K.S., Mi X. Experimental and computational surface hydrophobicity analysis of a non-enveloped virus and proteins. Colloids Surfaces B Biointerfaces. 2017;153:77–84. doi: 10.1016/j.colsurfb.2017.02.011. [DOI] [PubMed] [Google Scholar]
- 69.Mi X., Bromley E.K., Joshi P.U., Long F., Heldt C.L. Virus Isoelectric Point Determination Using Single-Particle Chemical Force Microscopy. Langmuir. 2020;36(1):370–378. doi: 10.1021/acs.langmuir.9b0307010.1021/acs.langmuir.9b03070.s001. [DOI] [PubMed] [Google Scholar]
- 70.Verbyla M.E., Mihelcic J.R. A review of virus removal in wastewater treatment pond systems. Water Res. 2015;71:107–124. doi: 10.1016/j.watres.2014.12.031. [DOI] [PubMed] [Google Scholar]
- 71.Németh Z., Szekeres Gergő.Péter., Schabikowski M., Schrantz K., Traber J., Pronk W., Hernádi K., Graule T. Enhanced virus filtration in hybrid membranes with MWCNT nanocomposite. R. Soc. Open Sci. 2019;6(1):181294. doi: 10.1098/rsos.181294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gutierrez L., Li X., Wang J., Nangmenyi G., Economy J., Kuhlenschmidt T.B., Kuhlenschmidt M.S., Nguyen T.H. Adsorption of rotavirus and bacteriophage MS2 using glass fiber coated with hematite nanoparticles. Water Res. 2009;43(20):5198–5208. doi: 10.1016/j.watres.2009.08.031. [DOI] [PubMed] [Google Scholar]
- 73.Mazurkow J.M., Yüzbasi N.S., Domagala K.W., Pfeiffer S., Kata D., Graule T. Nano-Sized Copper (Oxide) on Alumina Granules for Water Filtration: Effect of Copper Oxidation State on Virus Removal Performance. Environ. Sci. Technol. 2020;54(2):1214–1222. doi: 10.1021/acs.est.9b0521110.1021/acs.est.9b05211.s001. [DOI] [PubMed] [Google Scholar]
- 74.Bradley I., Straub A., Maraccini P., Markazi S., Nguyen T.H. Iron oxide amended biosand filters for virus removal. Water Res. 2011;45(15):4501–4510. doi: 10.1016/j.watres.2011.05.045. [DOI] [PubMed] [Google Scholar]
- 75.Domagała K., Jacquin C., Borlaf M., Sinnet B., Julian T., Kata D., Graule T. Efficiency and stability evaluation of Cu2O/MWCNTs filters for virus removal from water. Water Res. 2020;179 doi: 10.1016/j.watres.2020.115879. [DOI] [PubMed] [Google Scholar]
- 76.Liga M.V., Maguire-Boyle S.J., Jafry H.R., Barron A.R., Li Q. Silica decorated TiO2 for virus inactivation in drinking water -simple synthesis method and mechanisms of enhanced inactivation kinetics. Environ. Sci. Technol. 2013;47(12):6463–6470. doi: 10.1021/es400196p. [DOI] [PubMed] [Google Scholar]
- 77.Mintova S., Jaber M., Valtchev V. Nanosized microporous crystals: emerging applications. Chem. Soc. Rev. 2015;44(20):7207–7233. doi: 10.1039/C5CS00210A. [DOI] [PubMed] [Google Scholar]
- 78.Awala H., Gilson J.-P., Retoux R., Boullay P., Goupil J.-M., Valtchev V., Mintova S. Template-free nanosized faujasite-type zeolites. Nat. Mater. 2015;14(4):447–451. doi: 10.1038/nmat4173. [DOI] [PubMed] [Google Scholar]
- 79.Ng E.-P., Chateigner D., Bein T., Valtchev V., Mintova S. Capturing ultrasmall EMT zeolite from template-free systems. Science. 2012;335(6064):70–73. doi: 10.1126/science:1214798. [DOI] [PubMed] [Google Scholar]
- 80.Georgieva V., Anfray C., Retoux R., Valtchev V., Valable S., Mintova S. Iron loaded EMT nanosized zeolite with high affinity towards CO2 and NO. Microporous Mesoporous Mater. 2016;232:256–263. doi: 10.1016/J.MICROMESO.2016.06.015. [DOI] [Google Scholar]
- 81.Losch P., Joshi H.R., Vozniuk O., Grünert A., Ochoa-Hernández C., Jabraoui H., Badawi M., Schmidt W. Proton Mobility, Intrinsic Acid Strength, and Acid Site Location in Zeolites Revealed by Varying Temperature Infrared Spectroscopy and Density Functional Theory Studies. J. Am. Chem. Soc. 2018;140(50):17790–17799. doi: 10.1021/jacs.8b1158810.1021/jacs.8b11588.s001. [DOI] [PubMed] [Google Scholar]
- 82.Chibani S., Medlej I., Lebègue Sébastien, Ángyán János.G., Cantrel L., Badawi M. Performance of CuII-, PbII-, and HgII-Exchanged Mordenite in the Adsorption of I2, ICH3, H2O, CO, ClCH3, and Cl2: A Density Functional Study. ChemPhysChem. 2017;18(12):1642–1652. doi: 10.1002/cphc.v18.1210.1002/cphc.201700104. [DOI] [PubMed] [Google Scholar]
- 83.Hessou E.P., Kanhounnon W.G., Rocca D., Monnier H., Vallières C., Lebègue S., Badawi M. Adsorption of NO, NO2, CO, H2O and CO2 over isolated monovalent cations in faujasite zeolite: a periodic DFT investigation. Theor. Chem. Acc. 2018;137:161. doi: 10.1007/s00214-018-2373-2. [DOI] [Google Scholar]
- 84.Jabraoui H., Khalil I., Lebègue Sébastien, Badawi M. Ab initio screening of cation-exchanged zeolites for biofuel purification. Mol. Syst. Des. Eng. 2019;4(4):882–892. doi: 10.1039/C9ME00015A. [DOI] [Google Scholar]
- 85.Khalil I., Jabraoui H., Lebègue Sébastien, Kim W.J., Aguilera L.-J., Thomas K., Maugé F., Badawi M. Biofuel purification: Coupling experimental and theoretical investigations for efficient separation of phenol from aromatics by zeolites. Chem. Eng. J. 2020;402:126264. doi: 10.1016/j.cej.2020.126264. [DOI] [Google Scholar]
- 86.Marega R., Prasetyanto E.A., Michiels C., De Cola L., Bonifazi D. Fast Targeting and Cancer Cell Uptake of Luminescent. 2016:1–11. doi: 10.1002/smll.201601447. [DOI] [PubMed] [Google Scholar]
- 87.Pang L., Farkas K., Bennett G., Varsani A., Easingwood R., Tilley R., Nowostawska U., Lin S. Mimicking filtration and transport of rotavirus and adenovirus in sand media using DNA-labeled, protein-coated silica nanoparticles. Water Res. 2014;62:167–179. doi: 10.1016/j.watres.2014.05.055. [DOI] [PubMed] [Google Scholar]
- 88.Marinescu G., Culita D.C., Romanitan C., Somacescu S., Ene C.D., Marinescu V., Negreanu D.G., Maxim C., Popa M., Marutescu L., Stan M., Chifiriuc C. Novel hybrid materials based on heteroleptic Ru(III) complexes immobilized on SBA-15 mesoporous silica as highly potent antimicrobial and cytotoxic agents. Appl. Surf. Sci. 2020;520 doi: 10.1016/j.apsusc.2020.146379. [DOI] [Google Scholar]
- 89.Qin Y., Wen Z., Zhang W., Chai J., Liu D., Wu S. Different roles of silica nanoparticles played in virus transport in saturated and unsaturated porous media. Environ. Pollut. 2020;259 doi: 10.1016/j.envpol.2019.113861. [DOI] [PubMed] [Google Scholar]
- 90.Jadhav S.A., Garud H.B., Patil A.H., Patil G.D., Patil C.R., Dongale T.D., Patil P.S. Recent advancements in silica nanoparticles based technologies for removal of dyes from water. Colloid Interface Sci. Commun. 2019;30 doi: 10.1016/j.colcom.2019.100181. [DOI] [Google Scholar]
- 91.Diagboya P.N.E., Dikio E.D. Silica-based mesoporous materials; emerging designer adsorbents for aqueous pollutants removal and water treatment. Microporous Mesoporous Mater. 2018;266:252–267. doi: 10.1016/j.micromeso.2018.03.008. [DOI] [Google Scholar]
- 92.Abeer M.M., Rewatkar P., Qu Z., Talekar M., Kleitz F., Schmid R., Lindén M., Kumeria T., Popat A. Silica nanoparticles: A promising platform for enhanced oral delivery of macromolecules. J. Control. Release. 2020;326:544–555. doi: 10.1016/j.jconrel.2020.07.021. [DOI] [PubMed] [Google Scholar]
- 93.Poeckh T., Lopez S., Fuller A.O., Solomon M.J., Larson R.G. Adsorption and elution characteristics of nucleic acids on silica surfaces and their use in designing a miniaturized purification unit. Anal. Biochem. 2008;373:253–262. doi: 10.1016/j.ab.2007.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Clemens H., Pang L., Morgan L.K., Weaver L. Attenuation of rotavirus, MS2 bacteriophage and biomolecule-modified silica nanoparticles in undisturbed silt loam over gravels dosed with onsite wastewater. Water Res. 2020;169 doi: 10.1016/j.watres.2019.115272. [DOI] [PubMed] [Google Scholar]
- 95.Liu M., Hata A., Katayama H., Kasuga I. Consecutive ultrafiltration and silica adsorption for recovery of extracellular antibiotic resistance genes from an urban river. Environ. Pollut. 2020;260 doi: 10.1016/j.envpol.2020.114062. [DOI] [PubMed] [Google Scholar]
- 96.Berts I., Fragneto G., Porcar L., Hellsing M.S., Rennie A.R. Controlling adsorption of albumin with hyaluronan on silica surfaces and sulfonated latex particles. J. Colloid Interface Sci. 2017;504:315–324. doi: 10.1016/j.jcis.2017.05.037. [DOI] [PubMed] [Google Scholar]
- 97.Bone S., Alum A., Markovski J., Hristovski K., Bar-Zeev E., Kaufman Y., Abbaszadegan M., Perreault F. Physisorption and chemisorption of T4 bacteriophages on amino functionalized silica particles. J. Colloid Interface Sci. 2018;532:68–76. doi: 10.1016/j.jcis.2018.07.107. [DOI] [PubMed] [Google Scholar]
- 98.Sun N., Deng C., Liu Y., Zhao X., Tang Y., Liu R., Xia Q., Yan W., Ge G. Optimization of influencing factors of nucleic acid adsorption onto silica-coated magnetic particles: Application to viral nucleic acid extraction from serum. J. Chromatogr. A. 2014;1325:31–39. doi: 10.1016/j.chroma.2013.11.059. [DOI] [PubMed] [Google Scholar]
- 99.Sun M., Wang H., Li X. Modification of cellulose microfibers by polyglutamic acid and mesoporous silica nanoparticles for Enterovirus 71 adsorption. Mater. Lett. 2020;277 doi: 10.1016/j.matlet.2020.128320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kim J., Lee H., Lee J.-Y., Park K.-H., Kim W., Lee J.H., Kang H.-J., Hong S.W., Park H.-J., Lee S., Lee J.-H., Park H.-D., Kim J.Y., Jeong Y.W., Lee J. Photosensitized Production of Singlet Oxygen via C60 Fullerene Covalently Attached to Functionalized Silica-coated Stainless-Steel Mesh: Remote Bacterial and Viral Inactivation. Appl. Catal. B Environ. 2020;270 doi: 10.1016/j.apcatb.2020.118862. [DOI] [Google Scholar]
- 101.Galdino F.E., Picco A.S., Sforca M.L., Cardoso M.B., Loh W. Effect of particle functionalization and solution properties on the adsorption of bovine serum albumin and lysozyme onto silica nanoparticles. Colloids Surfaces B Biointerfaces. 2020;186 doi: 10.1016/j.colsurfb.2019.110677. [DOI] [PubMed] [Google Scholar]
- 102.Ghosh S., Vandana V. Nano-structured mesoporous silica/silver composite: Synthesis, characterization and targeted application towards water purification. Mater. Res. Bull. 2017;88:291–300. doi: 10.1016/j.materresbull.2016.12.044. [DOI] [Google Scholar]
- 103.Kurtz-Chalot A., Villiers C., Pourchez J., Boudard D., Martini M., Marche P.N., Cottier M., Forest V. Impact of silica nanoparticle surface chemistry on protein corona formation and consequential interactions with biological cells. Mater. Sci. Eng. C. 2017;75:16–24. doi: 10.1016/j.msec.2017.02.028. [DOI] [PubMed] [Google Scholar]
- 104.Chen Z., Hsu F.-C., Battigelli D., Chang H.-C. Capture and release of viruses using amino-functionalized silica particles. Anal. Chim. Acta. 2006;569:76–82. doi: 10.1016/j.aca.2006.03.103. [DOI] [Google Scholar]
- 105.He K., Chen C., Liang C., Liu C., Yang B., Chen X., Cai C. Highly selective recognition and fluorescent detection of JEV via virus-imprinted magnetic silicon microspheres. Sensors Actuators B Chem. 2016;233:607–614. doi: 10.1016/j.snb.2016.04.127. [DOI] [Google Scholar]
- 106.Zhan S., Yang Y., Shen Z., Shan J., Li Y., Yang S., Zhu D. Efficient removal of pathogenic bacteria and viruses by multifunctional amine-modified magnetic nanoparticles. J. Hazard. Mater. 2014;274:115–123. doi: 10.1016/j.jhazmat.2014.03.067. [DOI] [PubMed] [Google Scholar]
- 107.Cademartiri R., Anany H., Gross I., Bhayani R., Griffiths M., Brook M.A. Immobilization of bacteriophages on modified silica particles. Biomaterials. 2010;31:1904–1910. doi: 10.1016/j.biomaterials.2009.11.029. [DOI] [PubMed] [Google Scholar]
- 108.Riccò R., Liang W., Li S., Gassensmith J.J., Caruso F., Doonan C., Falcaro P. Metal-Organic Frameworks for Cell and Virus Biology: A Perspective. ACS Nano. 2018;12(1):13–23. doi: 10.1021/acsnano.7b08056. [DOI] [PubMed] [Google Scholar]
- 109.Doonan C., Riccò R., Liang K., Bradshaw D., Falcaro P. Metal-Organic Frameworks at the Biointerface: Synthetic Strategies and Applications. Acc. Chem. Res. 2017;50(6):1423–1432. doi: 10.1021/acs.accounts.7b00090. [DOI] [PubMed] [Google Scholar]
- 110.Adil K., Belmabkhout Y., Pillai R.S., Cadiau A., Bhatt P.M., Assen A.H., Maurin G., Eddaoudi M. Gas/vapour separation using ultra-microporous metal-organic frameworks: Insights into the structure/separation relationship. Chem. Soc. Rev. 2017;46(11):3402–3430. doi: 10.1039/C7CS00153C. [DOI] [PubMed] [Google Scholar]
- 111.Chibani S., Badawi M., Loiseau T., Volkringer C., Cantrel L., Paul J.-F. A DFT study of RuO4 interactions with porous materials: Metal-organic frameworks (MOFs) and zeolites. Phys. Chem. Chem. Phys. 2018;20(24):16770–16776. doi: 10.1039/C8CP01950A. [DOI] [PubMed] [Google Scholar]
- 112.Liang K., Richardson J.J., Cui J., Caruso F., Doonan C.J., Falcaro P. Biomimetics: Metal-Organic Framework Coatings as Cytoprotective Exoskeletons for Living Cells. Adv. Mater. 2016;28:8066. doi: 10.1002/adma.201670256. [DOI] [PubMed] [Google Scholar]
- 113.Xie B.-P., Qiu G.-H., Hu P.-P., Liang Z., Liang Y.-M., Sun B., Bai L.-P., Jiang Z.-H., Chen J.-X. Simultaneous detection of Dengue and Zika virus RNA sequences with a three-dimensional Cu-based zwitterionic metal–organic framework, comparison of single and synchronous fluorescence analysis. Sensors Actuators B Chem. 2018;254:1133–1140. doi: 10.1016/j.snb.2017.06.085. [DOI] [Google Scholar]
- 114.Zhang Y.-W., Liu W.-S., Chen J.-S., Niu H.-L., Mao C.-J., Jin B.-K. Metal-organic gel and metal-organic framework based switchable electrochemiluminescence RNA sensing platform for Zika virus. Sensors Actuators B Chem. 2020;321 doi: 10.1016/j.snb.2020.128456. [DOI] [Google Scholar]
- 115.Jia Z., Ma Y., Yang L., Guo C., Zhou N., Wang M., He L., Zhang Z. NiCo2O4 spinel embedded with carbon nanotubes derived from bimetallic NiCo metal-organic framework for the ultrasensitive detection of human immune deficiency virus-1 gene. Biosens. Bioelectron. 2019;133:55–63. doi: 10.1016/j.bios.2019.03.030. [DOI] [PubMed] [Google Scholar]
- 116.Yang J., Feng W., Liang K., Chen C., Cai C. A novel fluorescence molecularly imprinted sensor for Japanese encephalitis virus detection based on metal organic frameworks and passivation-enhanced selectivity. Talanta. 2020;212 doi: 10.1016/j.talanta.2020.120744. [DOI] [PubMed] [Google Scholar]
- 117.Clark K.J., Sarr A.B., Grant P.G., Phillips T.D., Woode G.N. In vitro studies on the use of clay, clay minerals and charcoal to adsorb bovine rotavirus and bovine coronavirus. Vet. Microbiol. 1998;63(2-4):137–146. doi: 10.1016/S0378-1135(98)00241-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Knappe M., Bodevin S., Selinka H.-C., Spillmann D., Streeck R.E., Chen X.S., Lindahl U., Sapp M. Surface-exposed amino acid residues of HPV16 L1 protein mediating interaction with cell surface heparan sulfate. J. Biol. Chem. 2007;282(38):27913–27922. doi: 10.1074/jbc.M705127200. [DOI] [PubMed] [Google Scholar]
- 119.Cagno V., Andreozzi P., D’Alicarnasso M., Jacob Silva P., Mueller M., Galloux M., Le Goffic R., Jones S.T., Vallino M., Hodek J., Weber J., Sen S., Janeček E.-R., Bekdemir A., Sanavio B., Martinelli C., Donalisio M., Rameix Welti M.-A., Eleouet J.-F., Han Y., Kaiser L., Vukovic L., Tapparel C., Král P., Krol S., Lembo D., Stellacci F. Broad-spectrum non-toxic antiviral nanoparticles with a virucidal inhibition mechanism. Nat. Mater. 2018;17(2):195–203. doi: 10.1038/nmat5053. [DOI] [PubMed] [Google Scholar]
- 120.Jones S.T., Cagno V., Janeček M., Ortiz D., Gasilova N., Piret J., Gasbarri M., Constant D.A., Han Y., Vuković L., Král P., Kaiser L., Huang S., Constant S., Kirkegaard K., Boivin G., Stellacci F., Tapparel C. Modified cyclodextrins as broad-spectrum antivirals. Sci. Adv. 2020;6(5):eaax9318. doi: 10.1126/sciadv.aax9318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kim S.K., Li Y.X. Medicinal benefits of sulfated polysaccharides from sea vegetables. Adv. Food Nutr. Res. 2011;64:391–402. doi: 10.1016/B978-0-12-387669-0.00030-2. [DOI] [PubMed] [Google Scholar]
- 122.Harhaji Trajković L.M., Mijatović S.A., Maksimović-Ivanić D.D., Stojanović I.D., Momčilović M.B., Tufegdžić S.J., Maksimović V.M., Marjanovi Žaklina.S., Stošić-Grujičić S.D. Anticancer properties of ganoderma lucidum methanol extracts in vitro and in vivo. Nutr. Cancer. 2009;61(5):696–707. doi: 10.1080/01635580902898743. [DOI] [PubMed] [Google Scholar]
- 123.Xu Y., Dong Q., Qiu H., Cong R., Ding K. Structural characterization of an arabinogalactan from Platycodon grandiflorum roots and antiangiogenic activity of its sulfated derivative. Biomacromolecules. 2010;11(10):2558–2566. doi: 10.1021/bm100402n. [DOI] [PubMed] [Google Scholar]
- 124.Wang L., Wang X., Wu H., Liu R. Overview on biological activities and molecular characteristics of sulfated polysaccharides from marine green algae in recent years. Mar. Drugs. 2014;12:4984–5020. doi: 10.3390/md12094984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Peng M.-H., Dai W.-P., Liu S.-J., Yu L.-W., Wu Y.-N., Liu R., Chen X.-L., Lai X.-P., Li X., Zhao Z.-X., Li G. Bioactive glycosides from the roots of Ilex asprella. Pharm. Biol. 2016;54(10):2127–2134. doi: 10.3109/13880209.2016.1146779. [DOI] [PubMed] [Google Scholar]
- 126.Akram M., Tahir I.M., Shah S.M.A., Mahmood Z., Altaf A., Ahmad K., Munir N., Daniyal M., Nasir S., Mehboob H. Antiviral potential of medicinal plants against HIV, HSV, influenza, hepatitis, and coxsackievirus: A systematic review. Phyther. Res. 2018;32(5):811–822. doi: 10.1002/ptr.v32.510.1002/ptr.6024. [DOI] [PubMed] [Google Scholar]
- 127.A.K. Singh, A.S. Bhadauria, P. Kumar, H. Bera, S. Saha, Bioactive and drug-delivery potentials of polysaccharides and their derivatives, in: S. Maiti, S. Jana (Eds.), Polysacch. Carriers Drug Deliv., Woodhead Publishing, 2019: pp. 19–48. doi:10.1016/B978-0-08-102553-6.00002-7.
- 128.Wijesinghe W.A.J.P., Jeon Y.-J. Biological activities and potential industrial applications of fucose rich sulfated polysaccharides and fucoidans isolated from brown seaweeds: A review. Carbohydr. Polym. 2012;88(1):13–20. doi: 10.1016/j.carbpol.2011.12.029. [DOI] [Google Scholar]
- 129.Prajapati V.D., Maheriya P.M., Jani G.K., Solanki H.K. Carrageenan: A natural seaweed polysaccharide and its applications. Carbohydr. Polym. 2014;105:97–112. doi: 10.1016/j.carbpol.2014.01.067. [DOI] [PubMed] [Google Scholar]
- 130.Seki Y., Mizukura M., Ichimiya T., Suda Y., Nishihara S., Masuda M., Takase-Yoden S. O-sulfate groups of heparin are critical for inhibition of ecotropic murine leukemia virus infection by heparin. Virology. 2012;424(1):56–66. doi: 10.1016/j.virol.2011.11.030. [DOI] [PubMed] [Google Scholar]
- 131.Liu J., Willför S., Xu C. A review of bioactive plant polysaccharides: Biological activities, functionalization, and biomedical applications. Bioact. Carbohydrates Diet. Fibre. 2015;5(1):31–61. doi: 10.1016/j.bcdf.2014.12.001. [DOI] [Google Scholar]
- 132.Faccin-Galhardi L.C., Aimi Yamamoto K., Ray S., Ray B., Carvalho Linhares R.E., Nozawa C. The in vitro antiviral property of Azadirachta indica polysaccharides for poliovirus. J. Ethnopharmacol. 2012;142(1):86–90. doi: 10.1016/j.jep.2012.04.018. [DOI] [PubMed] [Google Scholar]
- 133.Ma F., Kong S., Tan H., Wu R., Xia B., Zhou Y., Xu H. Structural characterization and antiviral effect of a novel polysaccharide PSP-2B from Prunellae Spica. Carbohydr. Polym. 2016;152:699–709. doi: 10.1016/j.carbpol.2016.07.062. [DOI] [PubMed] [Google Scholar]
- 134.Richards J.T., Kern E.R., Glasgow L.A., Overall J.C., Deign E.F., Hatch M.T. Antiviral activity of extracts from marine algae. Antimicrob. Agents Chemother. 1978;14(1):24–30. doi: 10.1128/AAC.14.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Witvrouw M., De Clercq E. Sulfated polysaccharides extracted from sea algae as potential antiviral drugs. Gen. Pharmacol. 1997;29(4):497–511. doi: 10.1016/S0306-3623(96)00563-0. [DOI] [PubMed] [Google Scholar]
- 136.Ono L., Wollinger W., Rocco I.M., Coimbra T.L.M., Gorin P.A.J., Sierakowski M.-R. In vitro and in vivo antiviral properties of sulfated galactomannans against yellow fever virus (BeH111 strain) and dengue 1 virus (Hawaii strain) Antiviral Res. 2003;60(3):201–208. doi: 10.1016/S0166-3542(03)00175-X. [DOI] [PubMed] [Google Scholar]
- 137.Hidari K.I.P.J., Takahashi N., Arihara M., Nagaoka M., Morita K., Suzuki T. Structure and anti-dengue virus activity of sulfated polysaccharide from a marine alga. Biochem. Biophys. Res. Commun. 2008;376(1):91–95. doi: 10.1016/j.bbrc.2008.08.100. [DOI] [PubMed] [Google Scholar]
- 138.Ghosh P., Adhikari U., Ghosal P.K., Pujol C.A., Carlucci María.J., Damonte E.B., Ray B. In vitro anti-herpetic activity of sulfated polysaccharide fractions from Caulerpa racemosa. Phytochemistry. 2004;65(23):3151–3157. doi: 10.1016/j.phytochem.2004.07.025. [DOI] [PubMed] [Google Scholar]
- 139.Mandal P., Pujol C.A., Carlucci María.J., Chattopadhyay K., Damonte E.B., Ray B. Anti-herpetic activity of a sulfated xylomannan from Scinaia hatei. Phytochemistry. 2008;69(11):2193–2199. doi: 10.1016/j.phytochem.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 140.Malagoli B.G., Cardozo F.T.G.S., Gomes J.H.S., Ferraz V.P., Simões C.M.O., Braga F.C. Chemical characterization and antiherpes activity of sulfated polysaccharides from Lithothamnion muelleri. Int. J. Biol. Macromol. 2014;66:332–337. doi: 10.1016/j.ijbiomac.2014.02.053. [DOI] [PubMed] [Google Scholar]
- 141.Ghosh T., Chattopadhyay K., Marschall M., Karmakar P., Mandal P., Ray B. Focus on antivirally active sulfated polysaccharides: From structure-activity analysis to clinical evaluation. Glycobiology. 2009;19:2–15. doi: 10.1093/glycob/cwn092. [DOI] [PubMed] [Google Scholar]
- 142.Heylen E., Neyts J., Jochmans D. Drug candidates and model systems in respiratory syncytial virus antiviral drug discovery. Biochem. Pharmacol. 2017;127:1–12. doi: 10.1016/j.bcp.2016.09.014. [DOI] [PubMed] [Google Scholar]
- 143.Dhama K., Karthik K., Khandia R., Munjal A., Tiwari R., Rana R., Khurana S.K., Sana Ullah, Khan R.U., Alagawany M., Farag M.R., Dadar M., Joshi S.K. Medicinal and Therapeutic Potential of Herbs and Plant Metabolites / Extracts Countering Viral Pathogens - Current Knowledge and Future Prospects. Curr. Drug Metab. 2018;19(3):236–263. doi: 10.2174/1389200219666180129145252. [DOI] [PubMed] [Google Scholar]
- 144.Yang J.-S., Xie Y.-J., He W. Research progress on chemical modification of alginate: A review. Carbohydr. Polym. 2011;84(1):33–39. doi: 10.1016/j.carbpol.2010.11.048. [DOI] [Google Scholar]
- 145.Damonte E., Matulewicz M., Cerezo A. Sulfated Seaweed Polysaccharides as Antiviral Agents. Curr. Med. Chem. 2012;11:2399–2419. doi: 10.2174/0929867043364504. [DOI] [PubMed] [Google Scholar]
- 146.Adhikari U., Mateu C.G., Chattopadhyay K., Pujol C.A., Damonte E.B., Ray B. Structure and antiviral activity of sulfated fucans from Stoechospermum marginatum. Phytochemistry. 2006;67(22):2474–2482. doi: 10.1016/j.phytochem.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 147.Copeland R., Balasubramaniam A., Tiwari V., Zhang F., Bridges A., Linhardt R.J., Shukla D., Liu J. Using a 3-O-sulfated heparin octasaccharide to inhibit the entry of herpes simplex virus type 1. Biochemistry. 2008;47(21):5774–5783. doi: 10.1021/bi800205t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sinha S., Astani A., Ghosh T., Schnitzler P., Ray B. Polysaccharides from Sargassum tenerrimum: Structural features, chemical modification and anti-viral activity. Phytochemistry. 2010;71(2-3):235–242. doi: 10.1016/j.phytochem.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 149.Tang W., Zou C., Da C., Cao Y., Peng H. A review on the recent development of cyclodextrin-based materials used in oilfield applications. Carbohydr. Polym. 2020;240 doi: 10.1016/j.carbpol.2020.116321. [DOI] [PubMed] [Google Scholar]
- 150.Mahjoubin-Tehran M., Kovanen P.T., Xu S., Jamialahmadi T., Sahebkar A. Cyclodextrins: Potential therapeutics against atherosclerosis. Pharmacol. Ther. 2020;214 doi: 10.1016/j.pharmthera.2020.107620. [DOI] [PubMed] [Google Scholar]
- 151.Irie T., Uekama K. Cyclodextrins in peptide and protein delivery. Adv. Drug Deliv. Rev. 1999;36:101–123. doi: 10.1016/S0169-409X(98)00057-X. [DOI] [PubMed] [Google Scholar]
- 152.Illum L. Nasal drug delivery: New developments and strategies. Drug Discov. Today. 2002;7(23):1184–1189. doi: 10.1016/S1359-6446(02)02529-1. [DOI] [PubMed] [Google Scholar]
- 153.Del Valle E.M.M. Cyclodextrins and their uses: A review. Process Biochem. 2004;39(9):1033–1046. doi: 10.1016/S0032-9592(03)00258-9. [DOI] [Google Scholar]
- 154.Ma X., Zhao Y. Biomedical Applications of Supramolecular Systems Based on Host-Guest Interactions. Chem. Rev. 2015;115(15):7794–7839. doi: 10.1021/cr500392w. [DOI] [PubMed] [Google Scholar]
- 155.Pinho E., Grootveld M., Soares G., Henriques M. Cyclodextrins as encapsulation agents for plant bioactive compounds. Carbohydr. Polym. 2014;101:121–135. doi: 10.1016/j.carbpol.2013.08.078. [DOI] [PubMed] [Google Scholar]
- 156.Zhou Y., Cheng G., Chen K., Lu J., Lei J., Pu S. Adsorptive removal of bisphenol A, chloroxylenol, and carbamazepine from water using a novel β-cyclodextrin polymer. Ecotoxicol. Environ. Saf. 2019;170:278–285. doi: 10.1016/j.ecoenv.2018.11.117. [DOI] [PubMed] [Google Scholar]
- 157.Chen B., Chen S., Zhao H., Liu Y., Long F., Pan X. A versatile Β-cyclodextrin and polyethyleneimine bi-functionalized magnetic nanoadsorbent for simultaneous capture of methyl orange and Pb(II) from complex wastewater. Chemosphere. 2019;216:605–616. doi: 10.1016/j.chemosphere.2018.10.157. [DOI] [PubMed] [Google Scholar]
- 158.Abdou S., Collomb J., Sallas F., Marsura A., Finance C. Beta-cyclodextrin derivatives as carriers to enhance the antiviral activity of an antisense oligonucleotide directed toward a coronavirus intergenic consensus sequence. Arch. Virol. 1997;142(8):1585–1602. doi: 10.1007/s007050050182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.De Clercq E. Antiviral therapy for human immunodeficiency virus infections. Clin. Microbiol. Rev. 1995;8(2):200–239. doi: 10.1128/CMR.8.2.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Moriya T., Kurita H., Matsumoto K., Otake T., Mori H., Morimoto M., Ueba N., Kunita N. Potent Inhibitory Effect of a Series of Modified Cyclodextrin Sulfates on the Replication of HIV-1 in Vitro. J. Med. Chem. 1992;35:4767. doi: 10.1021/jm00103a016. [DOI] [PubMed] [Google Scholar]
- 161.Moriya T., Saito K., Kurita H., Matsumoto K., Otake T., Mori H., Morimoto M., Ueba N., Kunita N. A New Candidate for an Anti-HIV-1 Agent: Modified Cyclodextrin Sulfate (mCDS71) J. Med. Chem. 1993;36(11):1674–1677. doi: 10.1021/jm00063a018. [DOI] [PubMed] [Google Scholar]
- 162.Otake T., Schols D., Witvrouw M., Naesens L., Nakashima H., Moriya T., Kurita H., Matsumoto K., Ueba N., De Clercq E. Modified cyclodextrin sulphates (mCDS11) have potent inhibitory activity against HIV and high oral bioavailability. Antivir. Chem. Chemother. 1994;5(3):155–161. doi: 10.1177/095632029400500303. [DOI] [Google Scholar]
- 163.Anand R., Nayyar S., Pitha J., Merril C.R. Sulphated Sugar Alpha-Cyclodextrin Sulphate, a Uniquely Potent Anti-HIV Agent, Also Exhibits Marked Synergism with AZT, and Lymphoproliferative Activity. Antivir. Chem. Chemother. 1990;1(1):41–46. doi: 10.1177/095632029000100107. [DOI] [Google Scholar]
- 164.Leydet A., Moullet Céline, Roque J.P., Witvrouw M., Pannecouque C., Andrei G., Snoeck R., Neyts J., Schols D., De Clercq E. Polyanion inhibitors of HIV and other viruses. 7. Polyanionic compounds and polyzwitterionic compounds derived from cyclodextrins as inhibitors of HIV transmission. J. Med. Chem. 1998;41(25):4927–4932. doi: 10.1021/jm970661f. [DOI] [PubMed] [Google Scholar]
- 165.Long L., Xue Y., Hu X., Zhu Y. Study on the influence of surface potential on the nitrate adsorption capacity of metal modified biochar. Environ. Sci. Pollut. Res. 2019;26(3):3065–3074. doi: 10.1007/s11356-018-3815-z. [DOI] [PubMed] [Google Scholar]
- 166.Hoog Antink M.M., Beutel S., Rezwan K., Maas M. Tailoring electrostatic surface potential and adsorption capacity of porous ceramics by silica-assisted sintering. Materialia. 2020;12:100735. doi: 10.1016/j.mtla.2020.100735. [DOI] [Google Scholar]
- 167.Cedergren M.I., Selbing A.J., Löfman O., Källen B.A.J. Chlorination byproducts and nitrate in drinking water and risk for congenital cardiac defects. Environ. Res. 2002;89(2):124–130. doi: 10.1006/enrs.2001.4362. [DOI] [PubMed] [Google Scholar]
- 168.Hijnen W.A.M., Beerendonk E.F., Medema G.J. Inactivation credit of UV radiation for viruses, bacteria and protozoan (oo)cysts in water: A review. Water Res. 2006;40(1):3–22. doi: 10.1016/j.watres.2005.10.030. [DOI] [PubMed] [Google Scholar]
- 169.Poornima Parvathi V., Umadevi M., Sasikala R., Parimaladevi R., Ragavendran V., Mayandi J., Sathe G.V. Novel silver nanoparticles/activated carbon co-doped titania nanoparticles for enhanced antibacterial activity. Mater. Lett. 2020;258:126775. doi: 10.1016/j.matlet.2019.126775. [DOI] [Google Scholar]
- 170.T. Motomura, Y. Miyashita, T. Ohwada, M. Onishi, N. Yamamoto, et al Motomura, T.M. Motomura Yuko; Ohwada, Takashi; Onishi; Makoto; Yamamoto, Naoki., Material for removing HIV and its related substances, Patent US5667684A, 1997.
- 171.Kadhom M., Deng B. Metal-organic frameworks (MOFs) in water filtration membranes for desalination and other applications. Appl. Mater. Today. 2018;11:219–230. doi: 10.1016/j.apmt.2018.02.008. [DOI] [Google Scholar]