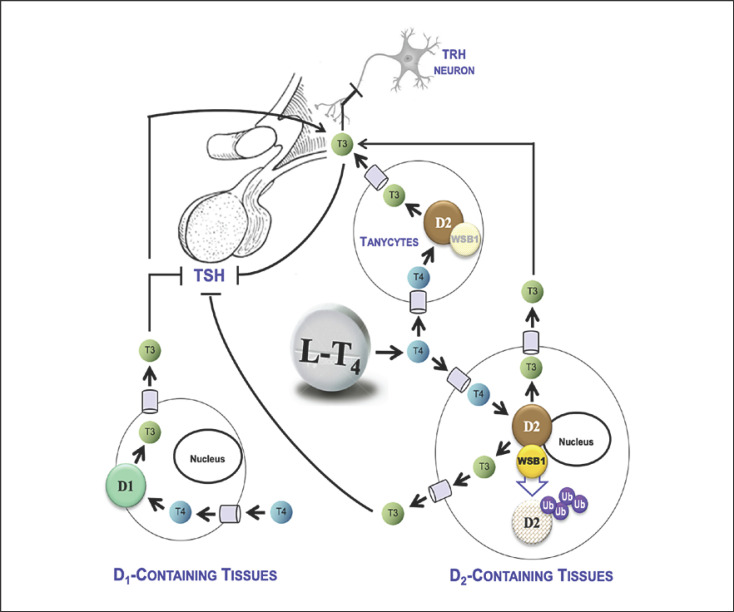
Fig. 1.
Relative roles of the D1 and D2 pathways in the TSH feedback mechanism during treatment with LT4. In LT4-treated thyroidectomized patients to achieve normal serum TSH levels, the D2 pathway contributes with ∼80% of the circulating T3 [6]. Studies performed in rats revealed how plasma T4 is taken up by the hypothalamic tanycytes and the pituitary thyrotrophs, and locally converted to T3 through the D2 pathway [8]. The net effect of the D2 activity in these two sites is a reduction in TSH secretion. As the goal of therapy with LT4 is to normalize serum TSH, a progressive increase in LT4 dose increases circulating T3 levels (predominantly through D2) and simultaneously reduces TSH secretion (predominantly through D2). Studies performed in rodents demonstrated that the D2 pathway is negatively regulated by T4, that is, D2 is ubiquitinated by WSB-1 and inactivated as it converts T4 to T3 [7]. However, this process occurs at a much slower rate in the hypothalamus [4]. Also in pituitary thyrotrophs, the loss of D2 activity caused by T4 is a much slower process [5]. As a result, D2-mediated T3 production is a more efficient process in the hypothalamus/pituitary unit when compared with other D2-containing tissues. Thus, while increasing the LT4 replacement dose to treat hypothyroidism, normalization of serum TSH levels will occur before full normalization of serum T3 levels. The role played by D1 is secondary, mainly because its affinity for T4 is three orders of magnitude less than D2, and its expression is positively regulated by plasma T3. Thus, D1 activity was never fully normalized in LT4-treated hypothyroid rats, only when a combination of LT4 and LT3 was used was serum T3 restored [4]. D1, type 1 deiodinase; D2, type 2 deiodinase; LT4, levothyroxine; T3, triiodothyronine; T4, thyroxine; TSH, thyrotropin; WSB-1, gene encoding the WD repeat and SOCS box-containing protein 1.