Figure 7a:
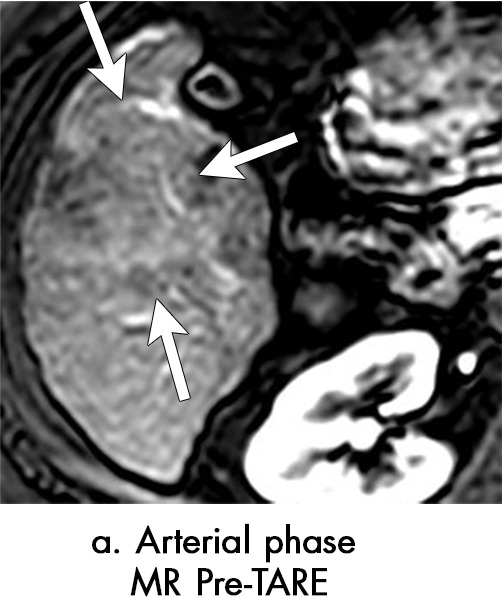
Axial MR images show various enhancement patterns after transarterial radioembolization (TARE) therapy in (a–d) a 56-year-old man, (e–f) an 82-year-old man, and (g–h) a 65-year-old woman. Geographic peritumoral arterial enhancement: (a) Pretreatment arterial phase MR image shows a 6.8-cm LI-RADS 5/OPTN 5× hepatocellular carcinoma (HCC). (b) Three months after TARE therapy, the tumor measured 7 cm with persistent central arterial phase hyperenhancement (mRECIST SD, LR-TR equivocal). (c) Six months after TARE therapy, the treated tumor was smaller with decreased central enhancement, but new peripheral geographic and nodular peritumoral hyperenhancement (arrowheads). These areas did not demonstrate washout on portal venous imaging (mRECIST PR, LR TR equivocal or nonviable). (d) Twelve months after TARE therapy, the tumor decreased in size with lack of central tumoral enhancement. There was persistent peripheral geographic and nodular peritumoral hyperenhancment, again without corresponding washout appearance. Note overlying hepatic capsular retraction, consistent with posttreatment parenchymal volume loss (mRECIST PR, LR-TR nonviable). Thin peritumoral ring of enhancement: (e) Pretreatment arterial phase MR image shows a 2.9-cm LI-RADS 5/OPTN 5b HCC. (f) Three months after TARE therapy there was a complete lack of central enhancement secondary to necrosis from 90Y therapy, with a smooth peritumoral ring of arterial enhancement (mRECIST PR, LR-TR nonviable). Complete nonenhancement: (g) Pretreatment arterial phase MR image shows a 6.2-cm LI-RADS 5/OPTN 5× HCC. (h) Three months after TARE therapy there was a decrease in size and central nonenhancement secondary to necrosis from 90Y therapy (mRECIST PR, LR TR nonviable). LR = LI-RADS, mRECIST = modified Response Evaluation Criteria for Solid Tumors, OPTN = Organ Procurement and Transplantation Network, PR = partial response, SD = stable disease, TR = treatment response.
