Abstract
Objectives
To assess the impact of two cycles of neoadjuvant chemotherapy (NAC) in patients who underwent nephroureterectomy for high‐risk cN0M0 upper tract urothelial carcinoma (UTUC), and to evaluate the efficacy of NAC in patients with localised disease (≤cT2).
Patients and Methods
We retrospectively analysed patients with high‐risk cN0M0 UTUC who received NAC followed by surgery, compared with a matched cohort who underwent initial surgery at Fujita Health University during 2005–2019. Baseline and tumour characteristics, overall survival (OS), cancer‐specific survival (CSS), and recurrence‐free survival (RFS) were compared between the cohorts. Cox proportional hazards models were used to identify predictors of survival.
Results
There were 117 and 67 patients in the study group and the control group, respectively. Significantly higher pathological downstaging (pDS) and lower lymphovascular invasion (LVI) were observed in the study group than in the control group (48% vs 22%, P = 0.008 and 29% vs 46%, P = 0.045, respectively). The NAC group had significantly better 5‐year OS (79% vs 53%, P = 0.003), 5‐year CSS (84% vs 66%, P = 0.008), and 5‐year RFS (80% vs 61%, P = 0.001) than the control group. The OS benefit of NAC was observed even in patients with localised (≤cT2) disease (P = 0.019). Patients with LVI showed significantly worse CSS both in pathologically locally advanced (≥pT3) and in localised (≤pT2) tumours (P = 0.048 and P = 0.018, respectively). Multivariate analysis identified LVI, NAC, and pDS as independent predictors of OS. Male sex and post‐NAC LVI were identified as predictors of worse survival in patients who underwent NAC.
Conclusions
Two cycles of NAC improved the survival of patients with high‐risk UTUC, even in patients with localised disease. Although two cycles of NAC appear to be effective in cN0M0 high‐risk UTUC including localised disease, additional larger sample size multicentre prospective studies comparing short‐course NAC regimens followed by surgery and surgery alone are required.
Keywords: nephroureterectomy, neoadjuvant therapy, urothelial carcinoma, lymphovascular invasion, preoperative chemotherapy, #utuc, #uroonc
Introduction
Upper tract urothelial carcinoma (UTUC) is a relatively rare malignancy and accounts for ~5–10% of all urothelial cancers [1, 2]. Because of its aggressiveness, ~60% of patients with UTUC present with muscle‐invasive disease at diagnosis, with 7% of these patients having metastasis [3]. The ‘gold standard’ treatment for high‐risk UTUC has been radical nephroureterectomy (RNU) with bladder cuff excision. However, oncological outcomes and therapeutic strategies have not been improved over the decades despite significant advances in the diagnosis, imaging, and surgical management of UTUC [4, 5, 6].
Although the role of perioperative chemotherapy in UTUC remains unclear, level 1 evidence has shown the survival benefit of neoadjuvant chemotherapy (NAC) in bladder cancer. Recently, the first Phase III randomised controlled trial (RCT) for UTUC reported the survival benefit of adjuvant chemotherapy after RNU [7]. Regarding NAC, well‐designed prospective trials are insufficient, whereas the Eastern Cooperative Oncology Group (ECOG)‐American College of Radiology Imaging Network (ACRIN) 8141 trial, a Phase II trial of NAC for extirpative surgery in patients with high‐grade UTUC, reported a 14% pathological complete response (pCR), although longer follow‐up is required to evaluate the survival outcomes [8]. Several retrospective studies have also shown that NAC induced pathological downstaging (pDS) and pCR associated with favourable oncological outcomes in high‐risk or locally advanced UTUC, supporting the consideration of NAC [9, 10, 11, 12, 13, 14]. Recent meta‐analyses have also shown that NAC was associated with higher rates of pDS and pCR and with better recurrence‐free survival (RFS), cancer‐specific survival (CSS) and overall survival (OS) in patients with locally advanced or node‐positive UTUC [15, 16]. On the contrary, the efficacy of NAC for localised or cN0M0 UTUC is still unclear.
The present study aimed to assess the effect of short cycles of NAC for patients with high‐risk cN0M0 UTUC at our institution. We hypothesised that NAC potentially improves the survival of patients with high‐risk UTUC not only with locally advanced tumour but also with localised disease.
Patients and Methods
Patients
We conducted a retrospective cohort study of 184 patients with high‐risk cN0M0 UTUC who underwent RNU at Fujita Health University during 2005–2019. High‐risk disease was defined as having any of the following conditions: hydronephrosis, tumour size of >2 cm, high‐grade cytology, high‐grade biopsy, multifocal disease, previous radical cystectomy for bladder cancer, and variant histology [1]. Patients’ demographic, renal function, surgical parameters, pathological parameters, and survival data were recorded in our database. Patients with clinically positive nodal disease, radiographic metastasis, ECOG Performance Status (ECOG PS) of >2 or estimated GFR (eGFR) of <30 mL/min/1.73 m2 were excluded from the analysis. The present study was approved by the local Institutional Review Board (authorisation number: HM20‐041).
NAC
NAC was performed according to the surgeon’s discretion and the patient’s consent. Two cycles of NAC were planned to avoid chemo‐related toxicity and delay of surgery. Patients with creatinine clearance of >50 mL/min received either gemcitabine 1000 mg/m2 on days 1, 8, and 15 plus cisplatin 70 mg/m2 (GCis) on day 2 every 4 weeks or standard dose of MVAC (methotrexate, 30 mg/m2, days 1, 15, and 22; vinblastine, 3 mg/m2, days 2, 15, and 22; doxorubicin, 30 mg/m2, day 2; and cisplatin, 70 mg/m2, day 2) in one to two cycles. Patients with creatinine clearance of 30–50 mL/min received gemcitabine 1000 mg/m2 on days 1 and 8 plus carboplatin (GCb) at an area under the curve of 4.5 according to the Calvert formula on day 2 every 3 weeks in one to two cycles. Toxicity of NAC was evaluated using the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE), version 4.0.
Surgical Procedure
All patients underwent RNU with kidney, ureter, and bladder cuff excision. Bladder cuff excision was performed using the extravesical approach. The surgical approach (open or laparoscopic) was based on the surgeon’s discretion. A sampling regional lymph node dissection was performed. To avoid delays in surgery, RNU was planned to be performed within 28 days after the completion of NAC.
Response Definitions
The role of response to NAC was evaluated in terms of pDS and pCR. pDS was defined as a pathological tumour stage that was at least one stage lower than the pre‐NAC clinical stage. pCR was defined as the absence of pathological evidence of urothelial carcinoma in the primary tumour.
Follow‐up
Regarding the follow‐up regimen, patients were evaluated every 3–6 months by performing a blood and serum test, urine cytology, cystoscopy, and CT to detect tumour recurrence. Recurrence was defined as tumour relapse in the operative field and presence of lymph node or distant metastasis.
Statistical Analyses
Categorical variables were compared using the Fisher’s exact test or chi‐square test. Quantitative variables were evaluated using the Student’s t‐test for normal distribution or the Mann–Whitney U‐test for non‐normal distribution. OS, CSS, and RFS were estimated using the Kaplan–Meier method and log‐rank test. The Cox proportional hazards regression model was used for uni‐ and multivariate analyses to identify the risk factors of mortality. To minimise selection bias, inverse probability of treatment weighting (IPTW)‐adjusted Cox regression analysis was further performed for predictors identified to be significant in multivariate analysis. Two‐sided P values of <0.05 were considered to indicate statistical significance. All statistical analyses were performed using GraphPad Prism 7.04 (GraphPad software, San Diego, CA, USA) and EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria) [17].
Results
Patient and Tumour Characteristics
We identified 184 patients with cN0M0 high‐risk UTUC who underwent RNU. The study group (NAC group) comprised 117 patients with UTUC who received a median of two cycles of platinum‐based NAC, followed by surgery. The control group (Ctrl group) comprised 67 patients who underwent initial surgery without NAC (Fig. S1A). Most patients (91.4%) received a cisplatin‐based regimen, and only 8.6% of cisplatin‐unfit patients received a carboplatin‐based regimen (Fig. S1B). NAC‐related adverse events were tolerable, and major severe adverse events (Grade ≥ 3) included neutropenia (41%), thrombocytopenia (13%), and anaemia (7%), as shown in Table S1. There were no significant differences between the two groups in baseline characteristics (Table S2). There was no significant difference in the eGFR before and after NAC. Moreover, the eGFR before surgery was not significantly different in the Ctrl and NAC groups (Fig. S1C). Significantly lower lymphovascular invasion (LVI) was observed in the NAC group (29%) than in the Ctrl group (46%) (Table 1). Five patients (4.3%) in the NAC group achieved pCR, whereas none of the patients in the Ctrl group (0%) achieved pCR. Additionally, pDS rate was significantly higher in the NAC group (48%) than in the Ctrl group (22%) (Table 1).
Table 1.
Tumour characteristics.
| Characteristic | Total (n = 184) | Ctrl (n = 67) | NAC (n = 117) | P |
|---|---|---|---|---|
| Clinical T stage, n (%) | ||||
| cTis/Ta | 5 (2.7) | 3 (4.5) | 2 (1.7) | 0.238 |
| cT1 | 62 (33.7) | 24 (35.8) | 38 (32.5) | |
| cT2 | 82 (44.6) | 28 (41.8) | 54 (46.2) | |
| cT3 | 35 (19.0) | 12 (17.9) | 23 (19.6) | |
| Pathological T stage, n (%) | ||||
| pT0 | 5 (2.7) | 0 (0) | 5 (4.3) | 0.185 |
| pTis/a | 35 (19.0) | 13 (19.4) | 22 (18.8) | |
| pT1 | 56 (30.4) | 16 (23.9) | 40 (34.2) | |
| pT2 | 36 (19.6) | 12 (17.9) | 24 (20.5) | |
| pT3 | 45 (24.5) | 23 (34.3) | 22 (18.8) | |
| pT4 | 7 (3.8) | 3 (4.5) | 4 (3.4) | |
| Pathological N stage, n (%) | ||||
| pN0 or Nx | 176 (95.6) | 64 (95.5) | 112 (95.7) | 0.713 |
| pN1 | 8 (4.4) | 3 (4.5) | 5 (4.3) | |
| Concomitant CIS, n (%) | ||||
| No | 168 (91.3) | 62 (92.4) | 106 (90.6) | 0.707 |
| Yes | 16 (8.7) | 5 (7.6) | 11 (9.4) | |
| LVI, n (%) | ||||
| No | 118 (64.1) | 36 (53.7) | 82 (70.1) | 0.045 |
| Yes | 66 (35.9) | 31 (46.3) | 35 (29.9) | |
| Surgical margins status, n (%) | ||||
| Negative | 177 (96.2) | 63 (94.0) | 114 (97.4) | 0.259 |
| Positive | 7 (3.8) | 4 (6.0) | 3 (2.6) | |
| Pathological downstaging, n (%) | ||||
| No | 113 (61.4) | 52 (77.6) | 61 (52.1) | 0.006 |
| Yes | 71 (38.6) | 15 (22.4) | 56 (47.9) | |
CIS, carcinoma in situ.
Survival Analysis
The median follow‐up periods were 51 months in the NAC group and 32 months in the Ctrl group. The NAC group had significantly better 5‐year OS (79% vs 53%, P = 0.003), 5‐year CSS (84% vs 66%, P = 0.008), and 5‐year RFS (80% vs 61%, P = 0.001) than the Ctrl group (Figs 1A,B and Fig. S2A). Additionally, patients with pDS had significantly better OS than patients with no pDS (93% vs 57%, P < 0.001) (Fig. 1C). Furthermore, patients without LVI had significantly better OS than patients with LVI (82% vs 53%, P < 0.001) (Fig. 1D). For the cT3 population, NAC was markedly beneficial to OS (70% vs 20%, P < 0.001) (Fig. 2A). Moreover, even in the clinically localised (≤cT2) cohort, the NAC group showed significantly better OS than the Ctrl group (81% vs 56%, P = 0.019) (Fig. 2B). In IPTW‐adjusted Cox regression analyses, LVI+ (hazard ratio [HR] 2.825, P = 0.019) was significantly associated with worse OS, and pDS (HR 0.375, P = 0.039) and NAC (HR 0.474, P = 0.042) were significantly associated with improved OS (Table 2). cT3 and LVI+ were significantly associated with worse RFS, and pDS and NAC were significantly associated with improved RFS (Table S3). LVI+, pDS and NAC were also identified as independent predictors of CSS (Table S4). We further performed multivariate analysis to identify the predictors for OS in patients who underwent NAC. In the analysis, neither the NAC regimen nor the number of cycles was significantly associated with OS, and male sex and LVI+ after NAC were considered as independent predictors of worse OS (Table 3). To further investigate the importance of LVI, we performed sub‐analyses assessing the association between LVI and pathological parameters and survival. The overall LVI+ rate was 37%, and LVI distributions in each group were 100%, 76%, 56%, and 11% in pT4, pT3, pT2, and pT1, respectively. The LVI+ rate in patients with pDS was 14%, which was significantly lower than that in patients without pDS (51%) (Table S5). Patients with LVI showed significantly worse CSS both in pathologically locally advanced (≥pT3) and in localised (≤pT2) tumours (P = 0.048 and P = 0.018, respectively) (Fig. 2C,D). A similar trend in RFS, although in the locally advanced group, did not show a statistically significant difference (Fig. S2B,C).
Fig. 1.
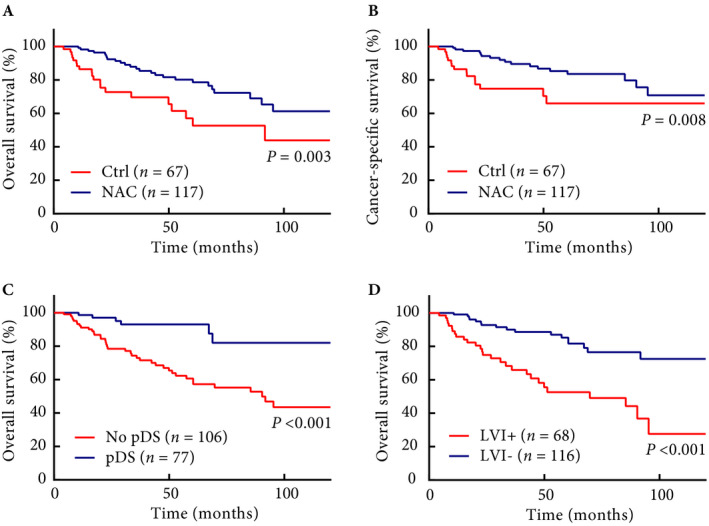
Oncological outcomes. (A) OS and (B) CSS in patients who received NAC or initial surgery without NAC. (C) OS in patients with pDS or without pDS. (D) OS in patients with and without LVI.
Fig. 2.
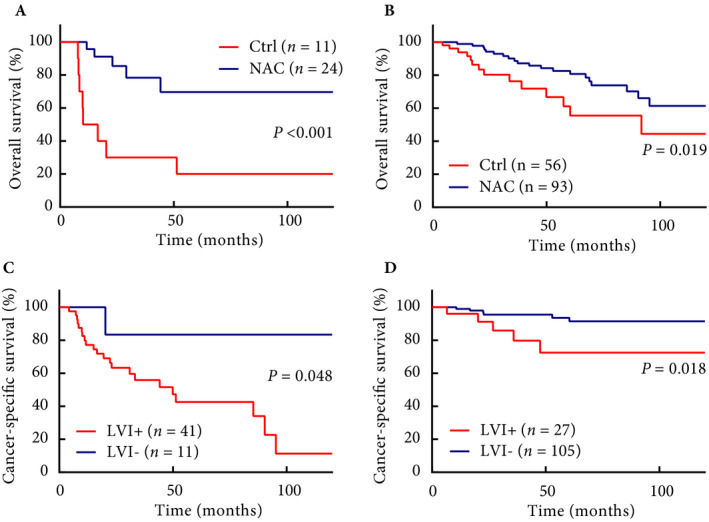
Oncological outcomes in subgroup analyses. (A) OS in patients with cT3 disease. (B) OS in patients with ≤cT2 disease. (C) CSS in patients with ≥pT3 disease. (D) CSS in patients with ≤pT2 disease.
Table 2.
Cox proportional hazard regression models for OS.
| Variable | Univariate | Multivariate | Multivariate (IPTW model) | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| Age | 1.037 (1.002–1.073) | 0.037 | 1.034 (0.998–1.070) | 0.061 | 1.032 (0.993–1.072) | 0.111 |
| Male sex | 1.295 (0.624–2.691) | 0.488 | 1.867 (0.876–3.979) | 0.105 | ||
| ECOG PS | ||||||
| 0 | 1 (reference) | 0.241 | ||||
| 1 | 1.943 (0.7649–4.937) | 0.163 | 2.009 (0.624–6.465) | |||
| Clinical T stage | ||||||
| ≤cT1 | 1 (reference) | |||||
| cT2 | 1.100 (0.546–2.216) | 0.790 | 0.887 (0.376–2.093) | 0.785 | ||
| cT3 | 2.570 (1.226–5.386) | 0.012 | 1.753 (0.741–4.148) | 0.201 | 1.571 (0.563–4.380) | 0.388 |
| Tumour location | ||||||
| Renal pelvis | 1 (reference) | |||||
| Ureter | 0.773 (0.430–1.387) | 0.388 | 1.076 (0.531–2.179) | 0.838 | ||
| Open approach | 1.869 (0.866–4.036) | 0.111 | 1.593 (0.659–3.854) | 0.302 | ||
| LVI+ | 3.659 (1.996–6.705) | <0.001 | 2.366 (1.080–5.187) | 0.032 | 2.825 (1.183–6.749) | 0.019 |
| pDS | 0.214 (0.091–0.508) | <0.001 | 0.359 (0.140–0.921) | 0.033 | 0.375 (0.145–0.958) | 0.039 |
| pCR | 0.688 (0.094–5.024) | 0.712 | ||||
| NAC | 0.417 (0.231–0.754) | 0.003 | 0.559 (0.306–0.946) | 0.043 | 0.474 (0.249–0.952) | 0.042 |
| AC | 1.562 (0.773–3.160) | 0.214 | ||||
AC, adjuvant chemotherapy.
Table 3.
Cox proportional hazard regression models for OS in patients who underwent NAC.
| Variable | Univariate | Multivariate | Multivariate (IPTW model) | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| Age | 1.028 (0.978–1.080) | 0.269 | 1.028 (0.972–1.087) | 0.331 | ||
| Male sex | 3.856 (0.908–16.37) | 0.067 | 5.816 (1.331–25.42) | 0.019 | ||
| ECOG PS | ||||||
| 0 | 1 (reference) | |||||
| 1 | 0.971 (0.130–7.239) | 0.977 | 2.411 (0.269–21.60) | 0.432 | ||
| Clinical T stage | ||||||
| cT1 or lower | 1 (reference) | |||||
| cT2 | 1.875 (0.751–4.679) | 0.178 | 1.129 (0.433–2.946) | 0.804 | ||
| cT3 | 2.317 (0.744–7.216) | 0.147 | 2.709 (0.719–10.21) | 0.141 | ||
| Tumor location | ||||||
| Renal pelvis | 1 (reference) | |||||
| Ureter | 0.557 (0.250–1.244) | 0.153 | 0.982 (0.385–2.503) | 0.969 | ||
| Open approach | 0.811 (0.189–3.466) | 0.777 | 0.413 (0.079–2.163) | 0.295 | ||
| LVI+ | 5.404 (2.352–12.42) | <0.001 | 3.987 (1.521–10.45) | 0.004 | 6.728 (2.527–17.91) | <0.001 |
| pDS | 0.317 (0.118–0.850) | 0.022 | 0.747 (0.242–2.299) | 0.611 | ||
| pCR | 0.932 (0.124–6.997) | 0.945 | ||||
| NAC regimen | ||||||
| MVAC | 1 (reference) | |||||
| GCis | 0.932 (0.362–2.400) | 0.8843 | ||||
| GCb | 1.668 (0.322–8.644) | 0.5420 | ||||
| No. NAC cycles | ||||||
| 1 | 1 (reference) | |||||
| 2 | 1.334 (0.553–3.216) | 0.526 | ||||
| AC | 2.103 (0.960–4.524) | 0.17 | ||||
AC, adjuvant chemotherapy.
Discussion
Recent studies have shown that NAC is associated with higher rates of pDS and pCR and improved RFS, CSS, and OS in patients with locally advanced or node‐positive UTUC [15, 16]. On the contrary, studies evaluating the role of NAC for localised or cN0M0 UTUC are insufficient [18, 19]. To the best of our knowledge, this is the first report to identify the prognostic factors of high‐risk UTUC after NAC, and the survival benefit of NAC for high‐risk localised disease.
NAC for UTUC is thought to have the potential to downstage tumours and eradicate occult micrometastases. We found that 48% of patients with pDS in the NAC group had significantly better OS, CSS, and RFS than patients in the Ctrl group. In terms of predicting the effects of NAC, pDS has been considered as an important surrogate marker [19]. The NAC group had a higher pDS rate than the Ctrl group, which was significantly associated with better survival in patients with locally advanced or node‐positive UTUC [10, 11, 14, 20]. These findings are consistent with our present results in terms of the efficacy of NAC for UTUC and the usefulness of pDS as a predictor for survival. However, there is a lack of accuracy and reliability in the definition of pDS depending on preoperative cross‐sectional imaging along with intra‐observer variability. Therefore, to minimise these biases for both the NAC and Ctrl groups, we recruited patients from the same time‐frame to normalise the temporal factors (Table S2). In addition, we used IPTW analysis to minimise the selection bias.
We found LVI as a valuable predictor of survival and NAC effect. A significantly lower incidence of LVI was observed in the NAC group than in the Ctrl group, which was identified as a robust predictor of OS, CSS, and RFS. LVI is defined as the presence of cancer cells within an endothelium‐lined space without underlying muscular walls and is considered an important step in tumour dissemination. In previous studies, LVI has been shown to significantly increase the risk of disease recurrence, cancer‐specific mortality, and overall mortality after RNU [21, 22, 23]. However, the association between the incidence of LVI and poor mortality was observed only in patients with node‐negative UTUC and not in patients with node‐positive UTUC. Thus, LVI has a higher predictive value in patients with localised or node‐negative disease than in patients with advanced disease with node or distant metastasis. In the present study, the positive effects of NAC on OS were observed even in patients with clinically localised cancer, suggesting the importance of NAC for localised disease. While assessing the association between LVI and pathological parameters, we observed that the incidence rate of LVI was significantly lower in the pDS+ group (14%) than in the pDS– group (51%). In addition, the LVI+ rate was significantly associated with RFS and CSS, even in patients with pathologically localised disease. Taken together, NAC appears to reduce LVI, which leads to better survival of not only the patients with locally advanced disease but also those with localised disease.
Three different outcome measures, pCR, pDS, and LVI reduction, indirectly reflected the pathological response induced by NAC, each factor having respective advantages and disadvantages. pCR is a robust endpoint of NAC as proved by the pathological findings regardless of the pre‐clinical stage. However, the rates of pCR have been reported as only 9.4–18% and no outcome measure in remaining patients without pCR. The present study demonstrated that pCR was not associated with patient survival, which might be attributed to the small number of patients who achieved pCR. In contrast, pDS can be easily evaluated using cross‐sectional imaging and is more frequently observed (27–44.5%) than pCR. Thus, several previous studies and the present study used this endpoint for evaluating the effect of NAC, despite the difficulty in accurate clinical staging, especially in localised disease. LVI is also a robust endpoint in localised disease as proven by pathological findings, although it is difficult to compare the pre‐ and post‐NAC LVI status. Taking into account the pDS rate, the difference between LVI status of the NAC group and the Ctrl group, and the impact of post‐NAC LVI status on prognosis, NAC appears to influence patient pathology and survival.
The optimal number of NAC cycles is not yet determined. It is notable that the NAC group, with two cycles of NAC, achieved higher pDS rate, lower LVI, and better survival than the Ctrl group. In previous studies that examined patients with node‐positive UTUC who underwent NAC, a median of six cycles of NAC was shown to achieve 27.8% pT0N0 rates, whereas two to four cycles of NAC did not result in pT0N0 (0%) [9, 20, 24]. These results suggested that more intensive NAC might be necessary to eradicate visible nodal disease. In contrast, in node‐negative or localised disease, although two cycles of NAC appears to be insufficient for total elimination of cancer cells and to achieve pCR, reduction of tumours and inhibition of LVI may be sufficient to maximise the effect of surgery and to improve survival. Short‐cycle NAC has the following advantages: it results in less chemo‐related toxicity, and in NAC, extirpative surgery is performed without delay, especially in chemo‐resistant patients. Several previous studies have reported survival benefit of a median of two cycles of NAC [9, 10, 11]. In addition, a recent study reported the prognostic impact of down‐grading of ipsilateral hydronephrosis using NAC in patients with ureteric carcinoma, and four out of nine patients achieved down‐grading of hydronephrosis using only one cycle of NAC [25]. These data supported the cytoreductive effect and survival benefit of the low number of NAC cycles, despite relatively low pCR rates.
In the present study, male sex was also identified as a predictor of worse survival after NAC. The factor was found to be significant only in the IPTW‐adjusted multivariate analysis performed to assess the OS in patients who received NAC, but not in the univariate analysis and other survival analyses. To date, the impact of sex on the survival rate of patients with UTUC or chemosensitivity remains controversial and seems to depend on race, smoking status, and tumour stage [26, 27, 28]. Further studies are needed to establish sex as a definitive predictor of NAC.
Our present study has several limitations. First, this was a single‐centre retrospective study with a relatively small sample size, which might result in selection biases and unmeasured confounders despite the recruitment of patients in the NAC group and the Ctrl group within the same time‐frame, and IPTW‐adjusted analysis. Second, the inaccuracy of preoperative staging or risk stratification based on cross‐sectional imaging and ureteroscopic biopsy was a major limitation of this study. Third, information on dissected lymph nodes was limited because lymph node dissection was not performed routinely, and thus, was not standardised. In addition, in the Ctrl and the NAC groups, 18% and 14% of patients received adjuvant chemotherapy, and 5% and 3% of patients received pembrolizumab, respectively, which possibly affected our survival analyses. Regardless of the aforementioned limitations, our present study suggested that NAC is significantly beneficial for high‐risk cN0M0 UTUC. To validate our present results, well‐designed prospective studies involving more patients are required. However, owing to the current imaging and biopsy techniques, regardless of the study design, the inaccuracy of clinical staging and pDS will remain.
The ongoing prospective RCTs, including the ECOG‐ACRIN 8141 trial, might drastically change the therapeutic strategy for UTUC in the near future. As post‐NAC LVI was identified as a predictor of worse OS, a novel treatment strategy, involving adjuvant therapies, including programmed death 1 (PD‐1), programmed death‐ligand 1 (PD‐L1), and fibroblast growth factor receptor (FGFR) inhibitors, is needed for the patients who experience LVI even after NAC. Further investigation of optimal systemic therapies including checkpoint inhibitors (CPIs) or tyrosine kinase inhibitors (TKIs) and preoperative therapy sequence is required to promote favourable clinical outcomes for patients with UTUC.
Conclusions
The NAC group achieved lower LVI and higher pDS that was significantly associated with better RFS, CSS, and OS, than the Ctrl group. The survival benefit was observed in both the cT3 and ≤cT2 cohorts. In the multivariate analyses, LVI, pDS, and NAC were significantly associated with RFS, CSS, and OS. NAC regimen and number of NAC cycles were not associated with OS in the NAC group. Male sex and incidence of LVI after NAC were considered as predictors of OS in patients who received NAC. Although short cycles of NAC appear to be effective in cN0M0 high‐risk UTUC including localised disease, the results of the ongoing prospective studies are required.
Conflict of Interest
None declared.
Abbreviations
- ACRIN
American College of Radiology Imaging Network
- CSS
cancer‐specific survival
- CTCAE
Common Terminology Criteria for Adverse Events
- ECOG (PS)
Eastern Cooperative Oncology Group (Performance Status)
- eGFR
estimated GFR
- GCb
gemcitabine, carboplatin
- GCis
gemcitabine, cisplatin
- HR
hazard ratio
- IPTW
inverse probability of treatment weighting
- LVI
lymphovascular invasion
- MVAC
methotrexate, vinblastine, doxorubicin, cisplatin
- NAC
neoadjuvant chemotherapy
- OS
overall survival
- pCR
pathological complete response
- pDS
pathological downstaging
- RCT
randomised controlled trial
- RFS
recurrence‐free survival
- RNU
radical nephroureterectomy
- UTUC
upper tract urothelial carcinoma
Supporting information
Fig. S1. Patient selection flow diagram, NAC regimen and perioperative renal function.
Fig. S2. Oncological outcomes in subgroup analyses.
Table S1. Neoadjuvant chemotherapy related adverse events (CTCAE, version 4.0).
Table S2. Patients’ characteristics.
Table S3. Cox proportional hazard regression models for recurrence‐free survival.
Table S4. Cox proportional hazard regression models for cancer‐specific survival.
Table S5. Association of LVI with pathological characteristics.
References
- 1. Rouprêt M, Babjuk M, Compérat E et al. European Association of urology guidelines on upper urinary tract urothelial carcinoma: 2017 update. Eur Urol 2018; 73: 111–22 [DOI] [PubMed] [Google Scholar]
- 2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019; 69: 7–34 [DOI] [PubMed] [Google Scholar]
- 3. Browne BM, Stensland KD, Moynihan MJ, Canes D. An analysis of staging and treatment trends for upper tract urothelial carcinoma in the national cancer database. Clin Genitourin Cancer 2018; 16: e743–50 [DOI] [PubMed] [Google Scholar]
- 4. Brown GA, Busby JE, Wood CG et al. Nephroureterectomy for treating upper urinary tract transitional cell carcinoma: time to change the treatment paradigm? BJU Int 2006; 98: 1176–80 [DOI] [PubMed] [Google Scholar]
- 5. Raman JD, Messer J, Sielatycki JA, Hollenbeak CS. Incidence and survival of patients with carcinoma of the ureter and renal pelvis in the USA, 1973–2005. BJU Int 2011; 107: 1059–64 [DOI] [PubMed] [Google Scholar]
- 6. Munoz JJ, Ellison LM. Upper tract urothelial neoplasms: incidence and survival during the last 2 decades. J Urol 2000; 164: 1523–5 [PubMed] [Google Scholar]
- 7. Birtle A, Johnson M, Chester J et al. Adjuvant chemotherapy in upper tract urothelial carcinoma (the POUT trial): a phase 3, open‐label, randomised controlled trial. Lancet 2020; 18 1268–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Margulis V, Puligandla M, Trabulsi EJ et al. Phase II trial of neoadjuvant systemic chemotherapy followed by extirpative surgery in patients with high grade upper tract urothelial carcinoma. J Urol 2020; 203: 690–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kitamura H, Igarashi M, Tanaka T et al. A role for preoperative systemic chemotherapy in node‐positive upper tract urothelial carcinoma treated with radical nephroureterectomy. Jpn J Clin Oncol 2012; 42: 1192–6 [DOI] [PubMed] [Google Scholar]
- 10. Hosogoe S, Hatakeyama S, Kusaka A et al. Platinum‐based neoadjuvant chemotherapy improves oncological outcomes in patients with locally advanced upper tract urothelial carcinoma. Eur Urol Focus 2018; 4: 946–53 [DOI] [PubMed] [Google Scholar]
- 11. Kubota Y, Hatakeyama S, Tanaka T et al. Oncological outcomes of neoadjuvant chemotherapy in patients with locally advanced upper tract urothelial carcinoma: a multicenter study. Oncotarget 2017; 24: 101500–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Almassi N, Gao T, Lee B et al. Impact of neoadjuvant chemotherapy on pathologic response in patients with upper tract urothelial carcinoma undergoing extirpative surgery. Clin Genitourin Cancer 2018; 16: e1237–42 [DOI] [PubMed] [Google Scholar]
- 13. Liao RS, Gupta M, Schwen ZR et al. Comparison of pathological stage in patients treated with and without neoadjuvant chemotherapy for high risk upper tract urothelial carcinoma. J Urol 2018; 200: 68–73 [DOI] [PubMed] [Google Scholar]
- 14. Foerster B, Abufaraj M, Petros F et al. Efficacy of preoperative chemotherapy in high risk upper tract urothelial carcinoma. J Urol 2020; 203: 1101–8 [DOI] [PubMed] [Google Scholar]
- 15. Quhal F, Mori K, Sari Motlagh R et al. Efficacy of neoadjuvant and adjuvant chemotherapy for localized and locally advanced upper tract urothelial carcinoma: a systematic review and meta‐analysis. Int J Clin Oncol 2020; 25:1037–54 [DOI] [PubMed] [Google Scholar]
- 16. Kim DK, Lee JY, Kim JW, Hah YS, Cho KS. Effect of neoadjuvant chemotherapy on locally advanced upper tract urothelial carcinoma: a systematic review and meta‐analysis. Crit Rev Oncol Hematol 2019; 135: 59–65 [DOI] [PubMed] [Google Scholar]
- 17. Kanda Y. Investigation of the freely available easy‐to‐use software 'EZR' for medical statistics. Bone Marrow Transplant 2013; 48: 452–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Porten S, Siefker‐Radtke AO, Xiao L et al. Neoadjuvant chemotherapy improves survival of patients with upper tract urothelial carcinoma. Cancer 2014; 15: 1794–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Martini A, Daza J, Poltiyelova E et al. Pathological downstaging as a novel endpoint for the development of neoadjuvant chemotherapy for upper tract urothelial carcinoma. BJU Int 2019; 124: 665–71 [DOI] [PubMed] [Google Scholar]
- 20. Kobayashi K, Saito T, Kitamura Y et al. Effect of preoperative chemotherapy on survival of patients with upper urinary tract urothelial carcinoma clinically involving regional lymph nodes. Int J Urol 2016; 23: 153–8 [DOI] [PubMed] [Google Scholar]
- 21. Kikuchi E, Margulis V, Karakiewicz PI et al. Lymphovascular invasion predicts clinical outcomes in patients with node‐negative upper tract urothelial carcinoma. J Clin Oncol 2009; 1: 612–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liu W, Sun L, Guan F, Wang F, Zhang G. Prognostic value of lymphovascular invasion in upper urinary tract urothelial carcinoma after radical nephroureterectomy: a systematic review and meta‐analysis. Dis Markers 2019; 2019: 7386140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Novara G, Matsumoto K, Kassouf W et al. Prognostic role of lymphovascular invasion in patients with urothelial carcinoma of the upper urinary tract: an international validation study. Eur Urol 2010; 57: 1064–71 [DOI] [PubMed] [Google Scholar]
- 24. Youssef RF, Shariat SF, Lotan Y et al. Upper urinary tract urothelial carcinoma with loco‐regional nodal metastases: insights from the Upper Tract Urothelial Carcinoma Collaboration. BJU Int 2011; 108: 1286–91 [DOI] [PubMed] [Google Scholar]
- 25. Miyake M, Marugami N, Fujiwara Y et al. Down‐grading of ipsilateral hydronephrosis by neoadjuvant chemotherapy correlates with favorable oncological outcomes in patients undergoing radical nephroureterectomy for ureteral carcinoma. Diagnostics (Basel) 2019; 23: 10. DOI: 10.3390/diagnostics10010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rink M, Ehdaie B, Cha EK et al. Stage‐specific impact of tumor location on oncologic outcomes in patients with upper and lower tract urothelial carcinoma following radical surgery. Eur Urol 2012; 62: 677–84 [DOI] [PubMed] [Google Scholar]
- 27. Huang CC, Su YL, Luo HL et al. Gender is a significant prognostic factor for upper tract urothelial carcinoma: a large hospital‐based cancer registry study in an endemic area. Front Oncol 2019; 9: 157. DOI: 10.3389/fonc.2019.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rink M, Xylinas E, Trinh QD et al. Gender‐specific effect of smoking on upper tract urothelial carcinoma outcomes. BJU Int 2013; 112: 623–37 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Patient selection flow diagram, NAC regimen and perioperative renal function.
Fig. S2. Oncological outcomes in subgroup analyses.
Table S1. Neoadjuvant chemotherapy related adverse events (CTCAE, version 4.0).
Table S2. Patients’ characteristics.
Table S3. Cox proportional hazard regression models for recurrence‐free survival.
Table S4. Cox proportional hazard regression models for cancer‐specific survival.
Table S5. Association of LVI with pathological characteristics.


