Abstract
Introduction
The aim is to test whether adding a simple physical test such as walking speed (WS) to the neuropsychological assessment increases the predictive ability to detect dementia.
Methods
The 2546 dementia‐free people from the SNAC‐K study were grouped into four profiles: (1) healthy profile; (2) isolated cognitive impairment, no dementia (CIND, scoring 1.5 standard deviation below age‐specific means on ≥1 cognitive domains); (3) isolated slow WS (<0.8 m/s); (4) CIND+ slow WS. The hazard of dementia (Cox regression), the positive and negative predictive values (PPV, NPV), and the area under the curve (AUC) were estimated.
Results
Participants with CIND +slow WS demonstrated the highest hazard of dementia (3.4; 95% confidence interval [CI]: 2.5–4.8). The AUC increased from 0.69 for isolated CIND to 0.83 for CIND+ slow WS. Such an increase was due to the improvement of the PPV, the NPV remaining optimal.
Discussion
Adding WS to the cognitive assessment dramatically increases the diagnostic accuracy of prodromal dementia.
Keywords: clinical markers, cognitive impairment, dementia, population‐based study, walking speed
1. INTRODUCTION
Cognitive impairment, no dementia (CIND) has been consistently shown to be strongly associated with further cognitive decline and to be a relevant at‐risk condition for incident dementia.1, 2 Consequently, CIND has the potential of becoming a useful clinical marker to identify older adults with a higher probability of developing dementia.3, 4 However, the actual significance of this cognitive stage, as well as several issues related to its progression, are not yet fully clarified.5 Many older individuals with CIND will remain cognitively stable or even revert to a normal state of cognition, with a reversion rate as high as 30%.6 Such heterogeneity is one of the major reasons for the low level of predictivity as concerns dementia. To overcome this limitation, research has recently focused on the identification of biomarkers that may help to increase the prediction of cognitive impairment.7 However, their costs and the necessity of a specialist clinical setting limit their applicability at population level. In other words, a clinically valid filter for dementia is still lacking.
Recently, some researchers have focused their attention on the physical functions of older people with cognitive impairment, suggesting that simple measurements such as walking speed (WS) may help in separate CIND individuals who will progress to dementia from those who will not.8, 9, 10, 11, 12 However, some questions remain unanswered: What is the added value of WS on top of the cognitive assessment in the prediction of dementia? Does its discriminative power decline over longer follow‐up? Strong evidence indicates WS as a clinical marker of accelerated aging13 and a good predictor of negative health‐related outcomes such as mortality and disability.14, 15 A number of studies have shown also that slow WS might predict poorer cognitive performance and subsequent dementia.16, 17, 18 Although largely an automatic movement and considered a relatively simple motor task, gait demands the coordination of different cortical and subcortical brain regions and networks and some researchers have shown that slower WS is strongly related to decreasing perceptual speed.13, 19 All these findings provide a good biologic rationale to the hypothesis that gait speed could be a useful complement to standard neuropsychological tests in the detection of prodromal dementia.20
In line with this hypothesis, over the last few years we have started to explore the body–mind connection in the development of dementia.21, 22, 23 In this study, focus is on both cognitive and physical impairments and the ability to detect individuals with prodromal dementia. The specific aims are (1) to quantify the hazard of dementia of isolated CIND, isolated slow WS, and their combination over a 12‐year period; and (2) to test whether adding WS to the neuropsychological assessment improves the predictive power of CIND as concerns dementia.
2. MATERIALS AND METHODS
2.1. Study population
We used data from the Swedish National Study on Aging and Care in Kungsholmen (SNAC‐K), an ongoing population‐based longitudinal study, detailed elsewhere.24 The study includes people aged 60+ living either at home or in institutions and resident in the Kungsholmen district of Stockholm. Of 5111 people randomly selected from 11 age cohorts, 73.3% (n = 3363) were examined. Participants have been followed up regularly, namely every 6 years (≤78 years) and every 3 years (78+).
People with prevalent dementia, severe vision or hearing problems that prevented them from undergoing the neuropsychological test battery, and those with missing data in the neuropsychological battery or in the WS test were excluded, and the final sample was 2546 participants. Of those, 538 took part only in the baseline assessment, due to death or refusal. Those participants were more likely to be older, female, with a lower level of education, and with a higher burden of diseases than the participants who showed at least two time‐point assessments (P < 0.001 for all).
The Regional Ethical Review Board in Stockholm, Sweden, approved the protocols of the SNAC‐K Study. All participants or next of kin (for cognitively impaired individuals) provided written informed consent.
The results follows the STROBE Recommendations.25
RESEARCH IN CONTEXT
Systematic review: After conducting a systematic review of the literature (PubMed and Web of Science) we retrieved three studies reporting a significant interplay between cognitive and physical impairments in the development of dementia, and two studies reporting negative results. Short follow‐ups, the utilization of subjective measures of cognitive impairment, and the involvement of samples non‐representative of the general population limit the comparability and generalizability of these—yet contrasting—results.
Interpretation: People presenting concurrently with cognitive and physical impairments experience the highest hazard of developing dementia over time. Adding walking speed to the cognitive battery improved the diagnostic accuracy of cognitive impairment in identifying incident dementia, suggesting that an easy‐to‐perform physical test reliably identifies patients who will progress to dementia.
Future directions: Studies on the biologic mechanisms underlying both cognitive and physical decline might open new research avenues for the prevention and treatment of dementia.
2.2. Data collection and definitions
In all waves, data were collected at the research center following standard procedures. At baseline and at each follow‐up assessment, nurses, physicians, and psychologists examined the participants and collected clinical, functional, laboratory, and cognitive data.
2.2.1. Cognitive impairment, no dementia
As previously described,12 we operationalized CIND when an objective impairment in cognition was detected (participants’ scores below 1.5 standard deviations [SDs] or more in age‐specific means in at least one cognitive domain), without meeting the diagnostic criteria of dementia.2 The following cognitive tests were administered: free recall (episodic memory), Trail Making Test Part B (executive function), Category and Letter Fluency (language), mental rotation (visuo‐spatial abilities), digit cancellation, and pattern comparison (perceptual speed). The raw scores were standardized into z scores using the baseline mean and SD of a random sample. In case of more than one test, we created the cognitive domain by averaging the z scores of the tests. The same procedure was used to create CIND at follow‐up, using the baseline cut‐offs.
2.2.2. Walking speed
Participants walked at usual pace over 6 or 2.4 m (if the individual reported to walk slowly or if space was restricted).19, 26 When the participant was unable to walk a value of zero was computed. We used a cutoff of <0.8 m/s to define slow WS.13, 27
2.2.3. Functional profiles
We grouped participants into four, mutually exclusive functional profiles based on the presence of CIND and slow WS: (1) healthy functional profile—without CIND and with a WS ≥ 0.8 m/s (reference group), (2) isolated CIND, (3) isolated slow WS, and (4) both CIND and slow WS. As sensitivity analyses, we used an alternative operationalization of cognitive impairment, by using a cutoff of 27 on the Mini Mental State Examination (MMSE), which is a commonly used screening tool in primary care and specialist clinical settings.28 Thus, we obtained the following four functional profiles: (1) healthy functional profile—MMSE ≥ 27 and WS ≥ 0.8 m/s (reference group), (2) isolated MMSE < 27, (3) isolated slow WS, and (4) both MMSE < 27 and slow WS.
2.2.4. Covariates
Data on age, sex, and education (highest level of formal education) were collected from the nurse's interview. A body mass index (BMI, kg/m2) <18.5 kg/m2 was considered indicative of malnutrition.29 Marital status was categorized as: partnered, widowed, unmarried, or divorced. Data from clinical interviews and examination, laboratory tests, use of medications, and inpatient and outpatient registers from the Swedish National Patient Register were used to identify chronic conditions,30 coded following the International Classification of Diseases, 10th edition. We considered cardio‐ and cerebrovascular diseases (ie, ischemic heart disease, heart failure, atrial fibrillation, diabetes, or stroke), chronic obstructive pulmonary disorders, solid neoplasms, and depression. As measure of global cognition we used the MMSE. DNA was extracted from peripheral blood samples and the apolipoprotein E (APOE) alleles were genotyped. Participants were categorized as ε4‐carriers (≥ε4 allele) and ε4‐no‐carriers.
2.2.5. Dementia diagnosis
The diagnosis of dementia was made both at baseline and at each follow‐up in accordance with the DSM‐IV‐TR,31 following a three‐step procedure. First, a preliminary diagnosis was made by the examining physician, followed by second preliminary diagnosis by a reviewing physician also involved in data collection. In cases of disagreement between the first and the second diagnoses, the final diagnosis was made by a neurologist who was external to the data‐collection process.3 For those participants who died between two follow‐up assessments, we complemented the diagnosis of dementia by: (1) linking the SNAC‐K database with the Swedish National Cause of Death Register, and (2) reviewing the clinical charts and medical records of the participants who died between two follow‐up assessments.
2.3. Statistical analysis
Multiplicative interaction between baseline CIND and slow WS was tested including both as indicator variables in the model (P for interaction <0.001). The association between the functional profiles and dementia was tested through Cox regression models. The proportional hazard assumption was assessed by regressing the scaled Schoenfeld residuals against survival time. No deviation from the proportional hazard assumption was detected. Because both the exposures and the outcome of this study are strongly influenced by age, age was used as time scale in the model. Adjusted hazard ratios (HRs) and 95% confidence interval (CI) were obtained. First, the functional profiles were considered only at baseline. Second, the same association was assessed by entering the functional profiles and the covariates into the model as time‐changing variables. This analysis assessed the studied association, accounting for the evolution of the exposure and the covariates over time.
Diagnostic accuracy measures (sensitivity, specificity, positive and negative predictive values [PPV, NPV], positive and negative likelihood ratios [LR+, LR−] and area under the curve [AUC] and their 95% CIs) were obtained for each functional profile, using the clinical diagnosis of dementia as reference standard within the first 6 years of follow‐up. Because age is strongly associated with both functional profiles and dementia, we tested the interaction between functional profiles and age (<78 and 78+ years, following the design of SNAC‐K) and we conducted stratified analyses (P for interaction <0.001).
2.3.1. Sensitivity analyses
The following sensitivity analyses for the survival and the prediction models were conducted. For the survival analysis: (1) we excluded the individuals with a MMSE<27 at baseline, (2) we repeated the analyses by using the MMSE to operationalize cognitive impairment, and (3) we performed a competing‐risk model to estimate the association between the functional profiles and dementia while considering death without dementia as a competing event. Concerning the accuracy measurements: (1) we repeated the analyses for the long‐term follow‐up (6–12 years) considering both only the baseline assessment of the functional profiles and their change within the first 6 years, (2) we used a different cutoff of slow WS (ie, 1 m/s), and (3) we repeated the analyses using the MMSE to operationalize cognitive impairment.
To test whether attrition might have affected the findings we repeated: (1) the survival analyses using competing risk regression models considering drop‐out status as a competing event and (2) the prediction models excluding those who dropped‐out. The proportion of missing covariates was 5.7% for APOE genotype; no other missing data have been detected. A complete data analysis was carried out on 94.3% of the cohort.
A P‐value <0.05 was considered statistically significant in all analyses. All analyses were performed using Stata version 14 (StataCorp, College Station, Texas, USA).
3. RESULTS
During the follow‐up (mean 8.5 years ± 4.0), of the 2546 people, 787 individuals died (n = 239 among the younger cohort; n = 548 among the older); 538 people dropped out (n = 330 among the younger cohorts; n = 208 among the older), and 193 incident dementia cases were identified. Among those who died between follow‐up assessments, by analysis of their clinical charts and the death register, 117 new dementia cases were retrieved. Overall, 310 incident dementia cases were identified (n = 78 among the younger cohorts; n = 232 among the older). Table S1 in supporting information shows the incident rate (IR) of dementia per 100 person years by functional profiles stratified by age. CIND+ slow WS had the following IR: 2.40 per 100 person‐years in the younger group and 7.68 per 100 person‐years in the older group. Incident cases were more likely to be women and with a high school educational level or below, older, with a greater number of chronic conditions, with a lower score at the MMSE, and were more likely to be carriers of the allele ε4 of APOE (p < 0.001 for all).
Figure S1 in supporting information depicts the study participation flow chart over the 12‐year follow‐up.
At study entry, the mean (±SD) age of the population was 72 (±9.9), 61% were female, and 14% had elementary‐level education. Sample baseline characteristics by functional profiles are shown in Table 1. People with co‐occurring CIND and slow WS were more likely to be older, women, to have a lower level of education, a greater number of chronic diseases, and a lower MMSE score than those in the healthy functional profile.
Table 1.
Sample baseline characteristics by functional profiles
| Functional profiles | |||||
|---|---|---|---|---|---|
| Healthy profile N = 1613 | Isolated CIND N = 441 | Isolated slow WS N = 255 | CIND + slow WS N = 237 | P value | |
| Women | 930 (57.7) | 275 (62.4) | 181 (71.0) | 175 (73.8) | <0.001 |
| Age (mean ± SD) | 69.3 ± 8.6 | 71.3 ± 8.6 | 81.1 ± 7.8 | 82.8 ± 9.4 | 0.049 |
| Education | |||||
| Elementary school | 143 (8.9) | 78 (17.7) | 50 (19.6) | 85 (35.9) | <0.001 |
| High school | 744 (46.1) | 244 (55.3) | 145 (56.9) | 114 (48.1) | |
| University | 726 (45.0) | 119 (27.0) | 60 (23.5) | 38 (16.0) | |
| Functional assessment | |||||
| MMSE score, (mean ± SD) | 29.2±1.0 | 28.5±1.7 | 28.4 ± 1.4 | 27.0 ± 2.6 | <0.001 |
| Walking speed (m/s) (mean ± SD) | 1.2 ± 0.3 | 1.1 ± 0.2 | 0.5 ± 0.2 | 0.4 ± 0.2 | <0.001 |
| Disability, at least one ADL impaired | 9 (0.6) | 3 (0.7) | 11 (4.4) | 41 (17.4) | <0.001 |
| Clinical assessment | |||||
| No. of chronic diseases (mean ± SD) | 3.1 ± 1.8 | 3.4 ± 1.8 | 5.0 ± 1.7 | 5.0 ± 1.8 | 0.707 |
| No. of medications (mean ± SD) | 3.1 ± 2.9 | 3.3 ± 2.9 | 5.9 ± 3.7 | 5.7 ± 3.6 | <0.001 |
| Hypertension | 1131 (70.1) | 344 (78.0) | 214 (83.9) | 202 (85.0) | <0.001 |
| Cardio‐ and cerebrovascular diseasesa | 338 (21.0) | 125 (28.3) | 123 (48.2) | 137 (57.8) | <0.001 |
| Depression | 46 (2.9) | 30 (6.8) | 19 (7.5) | 25 (10.7) | <0.001 |
| Solid neoplasms | 133 (8.3) | 30 (6.8) | 32 (12.6) | 28 (11.8) | 0.019 |
| Chronic obstructive pulmonary disorders | 53 (3.3) | 17 (3.9) | 28 (11.0) | 18 (7.6) | <0.001 |
| Malnutritionb | 21 (1.3) | 8 (1.8) | 14 (5.5) | 15 (6.3) | <0.001 |
| Genetic assessment | |||||
| APOE ε4 carrierc | 457 (29.6) | 134 (32.5) | 57 (24.0) | 54 (26.5) | 0.102 |
Note: Unless otherwise specified, figures show number (%). P‐values were obtained through chi‐squared test for categorical and analysis of variance for continuous variables.
Abbreviations: SD, standard deviation; CIND, cognitive impairment, no dementia; WS, walking speed. ADL, activities of daily living (analysis conducted on a sample of 2542).
Defined as ischemic heart disease, heart failure, diabetes, atrial fibrillation, or stroke.
Malnutrition, defined as body mass index <18.5 kg/m2.
Conducted on a sample of 2400 participants (94%).
Figure 1 shows the association between the functional profiles and dementia. In a fully adjusted model (for sex, education, chronic diseases, malnutrition, and APOE genotype) with age as time scale and considering the functional profiles and the covariates only at baseline, those with co‐occurring CIND and slow WS showed more than a three‐fold higher risk of dementia (HR: 3.4; 95% CI: 2.5−4.8) compared to those in the healthy functional profile. Isolated CIND was associated with a two‐fold higher risk of dementia (HR: 2.2; 95% CI: 1.6−3.0); no statistically significant association was detected between the isolated slow WS profile and dementia (HR: 1.2; 95% CI: 0.8−1.7). Consistent but attenuated results were obtained when we excluded those with a MMSE <27 (HR for isolated CIND: 2.0; 95% CI: 1.4−2.8; HR for isolated slow WS: 1.0; 95% CI: 0.7−1.6; HR for combined CIND and slow WS: 2.7; 95% CI: 1.8−4.1).
Figure 1.
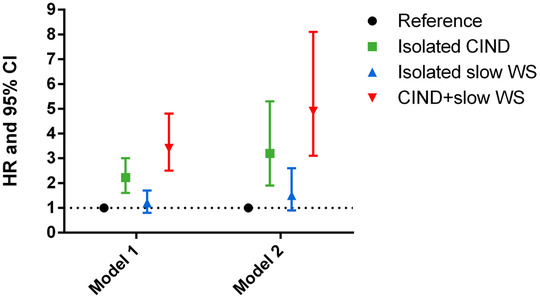
Hazard ratios (HR) of dementia with 95% confidence intervals by functional profiles. Hazard models considering age as time scale and adjusted for sex, education, malnutrition, chronic diseases, marital status, and APOE genotype. Abbreviations: CIND, cognitive impairment, no dementia; WS, walking speed; HR, hazard ratio; CI, confidence interval. *Participants without CIND and with a WS ≥ 0.8 m/s. Model 1: Considering the functional profiles and covariates at baseline only. Model 2: Considering the functional profiles and covariates as time‐changing variables
Consistent but stronger results were found when the functional profiles were considered time‐changing variables. People with both CIND and slow WS showed a five‐fold higher risk of dementia (HR: 4.9; 95% CI: 3.1–8.1), those with isolated CIND had a three‐fold higher risk of dementia (HR: 3.2; 95% CI: 1.9–5.3), and people with isolated slow WS had 50% increased risk of dementia (HR: 1.50; 95% CI: 0.9–2.6), although not statistically significant, with respect to the reference group. When the analyses were repeated excluding those with a MMSE lower than 27 at baseline, similar but attenuated results were found (HR for isolated CIND: 2.6; 95% CI: 1.6–4.1; HR for isolated slow WS: 1.9; 95% CI: 1.2–3.1; HR for combined CIND and slow WS: 4.0; 95% CI: 2.4–6.6).
Figure 2 depicts the predictive accuracy of functional profiles (AUC: area under the ROC curve) as concerns the identification of dementia within the first 6 years of follow‐up. Table S2 in supporting information shows the diagnostic accuracy measurements of the different functional profiles. All the accuracy measurements became stronger when CIND and slow WS were combined, achieving the best AUC. The presence of isolated CIND was more accurate in predicting incident dementia among the younger cohort with an AUC of 0.75 (95% CI: 0.61–0.88), while the AUC for isolated CIND in the older group was 0.70 (95% CI: 0.62–0.77). In the younger cohort, adding WS led to a minor improvement in the AUC in the prediction of dementia (0.76; 95% CI: 0.59–0.94); conversely, in the older cohort a statistically significantly higher AUC was detected when CIND was combined with slow WS (0.82; 95% CI: 0.76–0.87). Such an increase was due to a significant improvement of the PPV (from10% to 51%), NPV remaining at an equally high level (over 98%). Table S3 in supporting information shows the diagnostic accuracy for dementia of the different profiles, using a cutoff of WS of 1.0 m/s. The accuracy in the prediction of dementia improved in the younger participants, but at the expense of specificity across age groups and in the overall population.
Figure 2.

Area under the curve (AUC) for dementia by functional profiles, in the entire population and by age groups
Concerning the AUC, consistent but attenuated results were obtained for the longer follow‐up (6–12 years), when the functional profiles were considered time changing during the first 6 years of observation (Table S4 in supporting information). Conversely, the single baseline assessment of the profiles did not predict dementia in the long run.
Regarding both the survival analysis and the prediction model, the direction and significance of the studied association remained similar, although slightly attenuated, when we used MMSE to operationalize cognitive impairment (Table S5 in supporting information).
The competing risk regression model in which we considered dying without dementia and drop‐out status as competing event, and the prediction analyses repeated excluding those people who dropped out, led to consistent results.
4. DISCUSSION
Two main conclusions can be drawn from the results of this study. First, people presenting with cognitive and physical impairments concurrently experience the highest hazard of developing dementia over time. Second, adding an easy‐to‐perform measurement of physical function—such as WS—to a standard cognitive battery can help clinicians identify patients who will develop dementia. Such results were particularly strong in the short term. A single assessment of cognition and WS was not sufficient to predict long‐term dementia. Adding gait speed to the standard cognitive battery did not improve predictive power as concerns the identification of dementia in the younger cohort. Conversely, in the older age group the assessment of gait speed in addition to cognitive tests significantly improved the diagnostic accuracy of the identification of dementia. The use of a less conservative definition of slow WS (ie, <1 m/s) improved the diagnostic accuracy in the prediction of dementia in the younger participants, but decreased the specificity of the test.
Our findings have important implications for clinical routines involving people presenting with cognitive impairment in both primary care and specialist settings and emphasize the importance of measuring early functional impairment at the time of the first signs and symptoms of cognitive impairment. Gait is a relatively simple and automatic task,18, 32 but it requires the integrity of several different organs and systems, with a major role played by the central nervous system.20, 33 It can be considered a fine measure of motor performance, sensitive to changes in the cognitive performance in early stages. Several studies have shown that motor slowing may be an early marker of functional decline,34 which precedes by many years—and predicts—the onset of mild cognitive impairment.16 To note, functional decline in social and occupational tasks is already part of the diagnostic process of dementia, but those deficits appear later in the course of the disease and, as such, are more likely to be the consequences rather than early predictors of the disease itself. Reflecting clinical and subclinical disorders and being associated with accelerated aging, frailty, and adverse outcomes, WS is a valid indicator of biologic age, and in accordance with a recent study it performs better than the mere count of chronic diseases in the prediction of several adverse outcomes.35, 36 In older adults, the presence of cognitive impairment is frequently the result of complex and interrelated factors, such as the presence of multiple chronic conditions and poor contextual factors (eg, social deprivation). Thus, assessing gait speed might capture some of the non‐neurological aspects that often contribute to the onset and the progression of cognitive symptoms in older adults.21 Some alternative physical tests usually performed in clinical settings exist (eg, hand‐grip strength, balance test); however, it may be argued that they may selectively assess a single motor function (eg, muscle strength), require instruments (eg, dynamometer to assess weakness) not always available in non‐geriatric settings, and may be affected by floor or ceiling effects (eg, balance tests).37 Gait speed, beyond being influenced by such measures (ie, strength, balance), is greatly influenced by the central nervous system21 and can be assessed by non‐professionals using only a walkway and a stopwatch, making gait speed an easy‐to‐perform, non‐invasive, and inexpensive test that can be easily incorporated into routine clinical practice. Our results are in line with the studies that identify the Motoric Cognitive Risk syndrome (ie, co‐occurrence of cognitive complaints and slow WS) as an at‐risk profile for developing dementia. Also, they complement the evidence coming from the Gait and Brain Study that suggests that the dual task test can be considered a reliable tool to detect incident dementia in people with mild cognitive impairment.9, 10 The biologic and structural basis of the dual task resides in the fact that motor control and some cognitive processes rely on common neural circuits and, as a result, challenging gait with a cognitive task provides valuable information on the motor–cognitive interface and allows the detection of even milder cognitive deficits.38 Using gait speed alone may sometimes be insufficient to detect these minor cognitive impairments, as indicated by Montero‐Odasso in a recent study conducted on people with mild cognitive impairment.10, 39 Interestingly, this study was able to uncover a greater dementia risk in people with cognitive impairment through the assessment of WS, which is even easier to evaluate than the dual task.
Several biologic mechanisms support these results. The bidirectional interaction between body and mind in the development of dementia, although not completely understood, is universally recognized,22, 40 at least in older people. Different pathways may link cognition with motor functions and the complex coexistence of damage in several organs and systems might be the most plausible explanation.21 On one hand, several brain areas and networks—including the prefrontal cortex, the hippocampus, and the basal ganglia—with crucial roles to play in higher level cognitive performance, are also essential for gait control, and damage in these areas might be associated with both impairments in cognition and physical functioning.19, 41 A greater neuropathological burden (ie, amyloid plaques and neurofibrillary tangles) has been found in cognitively intact people with slow gait speed, suggesting that a slower WS might be an early marker of cognitive dysfunction.32, 42, 43 However, a high brain burden cannot usually completely explain the clinical picture.44 It is indeed well known that amyloid deposition and brain atrophy do not linearly correlate with cognition and functional decline, suggesting that other factors are implicated in the physiopathology of dementia.45, 46 A greater cardiovascular and metabolic burden together with a systemic inflammation status is likely to co‐occur and mixed pathology in older adults is the most plausible subtype.22, 47 This is in line with the idea that dementia in older adults might be considered a complex disease of aging rather than linked only to a neurodegenerative process.46, 48 Following this concept, gait speed may be considered a surrogate for such age‐related complexity and might serve clinicians in capturing a frailer subgroup of people.
4.1. Strengths and limitations
The results of this study come from a well‐characterized, large‐scale population‐based study with a 12‐year follow‐up. These aspects, together with the clinical assessment made by physicians and nurses, the extensive neuropsychological battery to assess cognitive performance, and the repeated measurement of functional profiles over time are the strengths—and the novelties—of this study. Moreover, few previous studies considered death as a competing risk for dementia, but we had the unique opportunity of identifying dementia cases also among those who died during the follow‐up assessments by performing a comprehensive review of clinical records and death registers, thus minimizing the influence of death on dementia incidence. Despite that, some milder cases might not have been captured, making it more likely that our results might have underestimated the association between the profiles and the dementia risk.49 Some limitations need to be acknowledged. First, the inclusion of participants with the neuropsychological battery only might have led to a selected study population. This, together with the fact that SNAC‐K participants are fit, well educated, and wealthy might have affected the generalizability of our findings. However, given that it was possible to characterize these people (older, female, and with a greater number of chronic diseases) our results are more likely to be an underestimation of the studied association. Finally, although the analyses were adjusted for major confounders and also the time variation of these confounders was taken into consideration in the analyses, the presence of residual confounding cannot be completely ruled out.
In conclusion, co‐occurring cognitive and motor impairments identify people with significantly higher dementia risk, suggesting that adding a simple measurement of physical function to the assessment of people with cognitive impairment may refine risk stratifications. In addition, it might help clinicians in the clinical management and care of people with cognitive problems. Biomarkers for Alzheimer's disease are increasingly promising, but are expensive and require specialist settings; consequently, a clinically valid marker is advocated. WS together with cognitive testing seems to meet such requirements. Future studies are needed to better understand the biologic mechanisms underlying both cognitive and physical decline to delineate new research lines for the prevention and treatment of dementia.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supporting information
Fig S1
Tables S1–S5
ACKNOWLEDGMENTS
We thank the SNAC‐K participants and the SNAC‐K group for their collaboration in data collection and management.
AUTHOR CONTRIBUTIONS
Giulia Grande, Debora Rizzuto, Davide L. Vetrano, and Laura Fratiglioni contributed to the conception and design of the study. Giulia Grande and Debora Rizzuto conducted the statistical analyses. All the authors contributed to the interpretation of results. Giulia Grande drafted the first version of the manuscript. All the authors critically revised the manuscript for important intellectual content. All the authors made a significant contribution to the research and the development of the manuscript and approved the final version for publication. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Grande G, Rizzuto D, Vetrano DL, et al. Cognitive and physical markers of prodromal dementia: A 12‐year‐long population study. Alzheimer's Dement. 2020;16:153–161. 10.1002/alz.12002
Funding information:
This work was supported by the funders of the Swedish National study on Aging and Care; the Ministry of Health and Social Affairs, Sweden; the participating county councils and municipalities; the Swedish Research Council; Karolinska Institutet (KID‐funding), Stockholm, Sweden. Swedish Research Council for Health, Working Life and Welfare.
REFERENCES
- 1. Caracciolo B, Palmer K, Monastero R, Winblad B, Backman L, Fratiglioni L. Occurrence of cognitive impairment and dementia in the community: a 9‐year‐long prospective study. Neurology. 2008;70:1778‐1785. [DOI] [PubMed] [Google Scholar]
- 2. Graham JE, Rockwood K, Beattie BL, et al. Prevalence and severity of cognitive impairment with and without dementia in an elderly population. Lancet. 1997;349:1793‐1796. [DOI] [PubMed] [Google Scholar]
- 3. Palmer K, Backman L, Winblad B, Fratiglioni L. Detection of Alzheimer's disease and dementia in the preclinical phase: population based cohort study. BMJ. 2003;326:245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Petersen RC. Clinical practice. Mild cognitive impairment. N Engl J Med. 2011;364:2227‐2234. [DOI] [PubMed] [Google Scholar]
- 5. Grande G, Cucumo V, Cova I, et al. Reversible mild cognitive impairment: the role of comorbidities at baseline evaluation. J Alzheimers Dis. 2016;51:57‐67. [DOI] [PubMed] [Google Scholar]
- 6. Canevelli M, Grande G, Lacorte E, et al. Spontaneous reversion of mild cognitive impairment to normal cognition: a systematic review of literature and meta‐analysis. J Am Med Dir Assoc. 2016;17:943‐948. [DOI] [PubMed] [Google Scholar]
- 7. Jack CR Jr, Bennett DA, Blennow K, et al. NIA‐AA research framework: toward a biological definition of Alzheimer's disease. Alzheimers Dement. 2018;14:535‐562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Doi T, Blumen HM, Verghese J, et al. Gray matter volume and dual‐task gait performance in mild cognitive impairment. Brain Imaging Behav. 2017;11:887‐898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Montero‐Odasso MM, Barnes B, Speechley M, et al. Disentangling cognitive‐frailty: results from the gait and brain study. J Gerontol A Biol Sci Med Sci. 2016;71:1476‐1482. [DOI] [PubMed] [Google Scholar]
- 10. Montero‐Odasso MM, Sarquis‐Adamson Y, Speechley M, et al. Association of dual‐task gait with incident dementia in mild cognitive impairment: results from the gait and brain study. JAMA Neurol. 2017;74:857‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Verghese J, Annweiler C, Ayers E, et al. Motoric cognitive risk syndrome: multicountry prevalence and dementia risk. Neurology. 2014;83:718‐726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Grande G, Vetrano DL, Fratiglioni L, et al. Disability trajectories and mortality in older adults with different cognitive and physical profiles. Aging Clin Exp Res. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA. 2011;305:50‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Newman AB, Simonsick EM, Naydeck BL, et al. Association of long‐distance corridor walk performance with mortality, cardiovascular disease, mobility limitation, and disability. JAMA. 2006;295:2018‐2026. [DOI] [PubMed] [Google Scholar]
- 15. Vetrano DL, Rizzuto D, Calderon‐Larranaga A, et al. Trajectories of functional decline in older adults with neuropsychiatric and cardiovascular multimorbidity: a Swedish cohort study. PLoS Med. 2018;15:e1002503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Buracchio T, Dodge HH, Howieson D, Wasserman D, Kaye J. The trajectory of gait speed preceding mild cognitive impairment. Arch Neurol. 2010;67:980‐986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Montero‐Odasso M, Oteng‐Amoako A, Speechley M, et al. The motor signature of mild cognitive impairment: results from the gait and brain study. J Gerontol A Biol Sci Med Sci. 2014;69:1415‐1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rosso AL, Metti AL, Faulkner K, et al. Complex walking tasks and risk for cognitive decline in high functioning older adults. J Alzheimers Dis. 2019;71:S65‐S73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Welmer AK, Rizzuto D, Qiu C, Caracciolo B, Laukka EJ. Walking speed, processing speed, and dementia: a population‐based longitudinal study. J Gerontol A Biol Sci Med Sci. 2014;69:1503‐1510. [DOI] [PubMed] [Google Scholar]
- 20. Tian Q, Chastan N, Bair WN, Resnick SM, Ferrucci L, Studenski SA. The brain map of gait variability in aging, cognitive impairment and dementia‐A systematic review. Neurosci Biobehav Rev. 2017;74:149‐162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Grande G, Triolo F, Nuara A, Welmer AK, Fratiglioni L, Vetrano DL. Measuring gait speed to better identify prodromal dementia. Exp Gerontol. 2019;124:110625. [DOI] [PubMed] [Google Scholar]
- 22. Qiu C, Fratiglioni L. A major role for cardiovascular burden in age‐related cognitive decline. Nat Rev Cardiol. 2015;12:267‐277. [DOI] [PubMed] [Google Scholar]
- 23. Grande G, Haaksma ML, Rizzuto D, et al. Co‐occurrence of cognitive impairment and physical frailty, and incidence of dementia: systematic review and meta‐analysis. Neurosci Biobehav Rev. 2019;107:96‐103. [DOI] [PubMed] [Google Scholar]
- 24. Lagergren M, Fratiglioni L, Hallberg IR, et al. A longitudinal study integrating population, care and social services data. The Swedish National study on Aging and Care (SNAC). Aging Clin Exp Res. 2004;16:158‐168. [DOI] [PubMed] [Google Scholar]
- 25. von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61:344‐349. [DOI] [PubMed] [Google Scholar]
- 26. Laukka EJ, Lovden M, Herlitz A, et al. Genetic effects on old‐age cognitive functioning: a population‐based study. Psychol Aging. 2013;28:262‐274. [DOI] [PubMed] [Google Scholar]
- 27. Stanaway FF, Gnjidic D, Blyth FM, et al. How fast does the Grim Reaper walk? Receiver operating characteristics curve analysis in healthy men aged 70 and over. BMJ. 2011;343:d7679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. O'Bryant SE, Humphreys JD, Smith GE, et al. Detecting dementia with the mini‐mental state examination in highly educated individuals. Arch Neurol. 2008;65:963‐967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cederholm T, Bosaeus I, Barazzoni R, et al. Diagnostic criteria for malnutrition ‐ An ESPEN Consensus Statement. Clin Nutr. 2015;34:335‐340. [DOI] [PubMed] [Google Scholar]
- 30. Calderon‐Larranaga A, Vetrano DL, Onder G, et al. Assessing and measuring chronic multimorbidity in the older population: a proposal for its operationalization. J Gerontol A Biol Sci Med Sci. 2016;72:1417‐1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Diagnostic and Statistical Manual on Mental Disorders , IV edition.
- 32. Rosso AL, Verghese J, Metti AL, et al. Slowing gait and risk for cognitive impairment: The hippocampus as a shared neural substrate. Neurology. 2017;89:336‐342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Drew T, Andujar JE, Lajoie K, Yakovenko S. Cortical mechanisms involved in visuomotor coordination during precision walking. Brain Res Rev. 2008;57:199‐211. [DOI] [PubMed] [Google Scholar]
- 34. Abe T, Kitamura A, Taniguchi Y, et al. Pathway from gait speed to incidence of disability and mortality in older adults: a mediating role of physical activity. Maturitas. 2019;123:32‐36. [DOI] [PubMed] [Google Scholar]
- 35. Zucchelli A, Vetrano DL, Grande G, et al. Comparing the prognostic value of geriatric health indicators: a population‐based study. BMC Med. 2019;17:185. 10.1186/s12916-019-1418-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rosano C, Studenski SA, Aizenstein HJ, Boudreau RM, Longstreth WT, Jr , Newman AB. Slower gait, slower information processing and smaller prefrontal area in older adults. Age Ageing. 2012;41:58‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Stijntjes M, Aartsen MJ, Taekema DG, et al. Temporal relationship between cognitive and physical performance in middle‐aged to oldest old people. J Gerontol A Biol Sci Med Sci. 2017;72:662‐668. [DOI] [PubMed] [Google Scholar]
- 38. Camicioli R, Howieson D, Lehman S, Kaye J. Talking while walking: the effect of a dual task in aging and Alzheimer's disease. Neurology. 1997;48:955‐958. [DOI] [PubMed] [Google Scholar]
- 39. Montero‐Odasso M, Bergman H, Phillips NA, Wong CH, Sourial N, Chertkow H. Dual‐tasking and gait in people with mild cognitive impairment. The effect of working memory. BMC Geriatr. 2009;9:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Qiu C, Winblad B, Fratiglioni L. The age‐dependent relation of blood pressure to cognitive function and dementia. Lancet Neurol. 2005;4:487‐99. [DOI] [PubMed] [Google Scholar]
- 41. Takakusaki K. Functional neuroanatomy for posture and gait control. J Mov Disord. 2017;10:1‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Del Campo N, Payoux P, Djilali A, et al. Relationship of regional brain beta‐amyloid to gait speed. Neurology. 2016;86:36‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rosso AL, Studenski SA, Chen WG, et al. Aging, the central nervous system, and mobility. J Gerontol A Biol Sci Med Sci. 2013;68:1379‐1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Villemagne VL, Pike KE, Chetelat G, et al. Longitudinal assessment of Abeta and cognition in aging and Alzheimer disease. Ann Neurol. 2011;69:181‐192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Beauchet O, Annweiler C, Montero‐Odasso M, Fantino B, Herrmann FR, Allali G. Gait control: a specific subdomain of executive function? J Neuroeng Rehabil. 2012;9:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wallace LMK, Theou O, Godin J, Andrew MK, Bennett DA, Rockwood K. Investigation of frailty as a moderator of the relationship between neuropathology and dementia in Alzheimer's disease: a cross‐sectional analysis of data from the Rush Memory and Aging Project. Lancet Neurol. 2019;18:177‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Haaksma ML, Vilela LR, Marengoni A, et al. Comorbidity and progression of late onset Alzheimer's disease: a systematic review. PLoS One. 2017;12:e0177044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wallace L, Theou O, Rockwood K, Andrew MK. Relationship between frailty and Alzheimer's disease biomarkers: a scoping review. Alzheimers Dement. 2018;10:394‐401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rizzuto D, Feldman AL, Karlsson IK, Dahl Aslan AK, Gatz M, Pedersen NL. Detection of dementia cases in two swedish health registers: a validation study. J Alzheimers Dis. 2018;61:1301‐1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Tables S1–S5


