Abstract
Background
Psychosocial wellbeing is an important determinant for patients' oral health‐related quality of life (OHRQoL). Psychosocial impact (PI), together with the dimensions Oral Function, Orofacial Pain and Orofacial Appearance, has been proposed to cover the different areas of OHRQoL.
Objective
The objective of the study was to collect further scientific support for the new four‐dimensional structure of OHRQoL. This study is one out of a series of four and focuses on the PI in patients with dental anxiety, oral cancer and periodontitis (PROSPERO registration number: CRD42017064033).
Methods
Five databases (Pubmed (Medline), EMBASE, Cochrane, CINAHL and PsycINFO) were electronically searched on 8 June 2017 and updated on 14 January 2019, to identify the studies that measure OHRQoL using the Oral Health Impact Profile (OHIP) for oral health conditions. In this review, studies were included if the mean/median domain scores from OHIP‐14 or OHIP‐49 were available for patients with dental anxiety, oral cancer or periodontitis. The score of the handicap domain from the OHIP was used to assess patients` PI. The handicap domain includes 6 items for OHIP‐49 with a domain score ranging from 0 to 24 and 2 items for OHIP‐14 with a domain score ranging from 0 to 8. For comparison between the 2 versions of the OHIP, the domain score of OHIP‐49 was conversed into a 0 to 8 metric. The domain scores of the included studies were then pooled, separately for each of the included dental disorders.
Results
A total of 2104 records were identified based on the search strategy. After screening of titles and abstracts, 1607 articles were reviewed in full text. Twenty‐three articles met the inclusion criteria for this review and were included in the study. The 23 articles contained 3884 patients, grouped in 30 patient populations and 42 patient samples. The pooled mean scores of PI for dental anxiety, oral cancer and periodontitis were 3.2, 1.9 and 0.8, respectively, on the 0 to 8 metric.
Conclusion
This review provides standardised information about the OHRQoL impact for three dental disorders as a model for the PI dimension. Dental anxiety tends to show the strongest effect on the PI dimension, while periodontitis tends to show the weakest effect on the PI dimension. Future studies need to confirm whether the reported differences in PI scores between the three dental disorders are statistically significant.
Keywords: dental anxiety, oral cancer, Oral Health‐Related Quality of Life, periodontitis, psychosocial impact
1. INTRODUCTION
In daily practice, a dentist needs to objectify a patient's complaints both on a physical level and on a psychosomatic level. Based on this information, a patient‐tailored treatment plan can be developed, taking into account patient characteristics that may interfere with healing and/or treatment adherence. 1 Clinicians in most (if not all) medical fields accept the biopsychosocial model as the most heuristic approach to understand and manage (chronic) pain and dysfunction. In this model, pain and disability interact with psychological and social factors, and these factors together determine the impact of a clinical condition on the individual patient. 2 In the field of dentistry, this dual‐axis approach is incorporated in the diagnostic classification for temporomandibular disorder patients 3 and could be applied to other pain conditions, including the various dental pain patients. 1 Options to measure psychological and social factors include questionnaires on specific constructs, like depression, 4 anxiety 5 or social support. 6
During the treatment process, the outcomes of treatment are continuously monitored and compared with the expectations for that treatment. Patient‐perceived impact of treatment nowadays is considered a highly important tool to evaluate treatment success. To measure the patient‐perceived impact consistently across different oral health conditions, the concept of Oral Health‐Related Quality of Life (OHRQoL) is widely acknowledged. The most commonly used questionnaire to measure OHRQoL is the Oral Health Impact Profile (OHIP). 7 The OHIP was originally developed and evaluated by Slade and Spencer in 1994. 7 Up to date, the OHIP has been translated into multiple languages and is further developed into several versions with a smaller number of items as compared to the original 49‐item of the OHIP, such as the 14‐item version. 7 , 8 In both OHIP‐14 and OHIP‐49, seven domains of OHRQoL are incorporated, covering functional limitation, physical pain, psychological discomfort, physical disability, psychological disability, social disability and handicap. The OHIP can be used to capture changes in health, with a proposed 7‐day recall period. 9 In clinical practice and research, both OHIP‐14 and OHIP‐49 are widely used for the assessment of OHRQoL in different target populations. However, OHIP‐14 is preferred to OHIP‐49 by researchers and clinicians because it is more practical due to less number of items, while it still has acceptable reliability, validity and precision. 8
Recent empirical data have shown that an approach using only four dimensions (ie Orofacial Pain, Orofacial Appearance, Oral Function and Psychosocial Impact) can serve as a more simple and clinically appealing set of OHRQoL dimensions and still provide a psychometrical accurate OHRQoL measurement. 10 In a recent systematic review, it was found that the four OHRQoL dimensions were the attributes that underlie all generic dental patient‐reported outcome measures. 11
In this special issue, the utility of the four‐dimensional structure of OHRQoL is evaluated. 10 In a series of four systematic reviews, one for each dimension, further evidence for the concept of these new dimensions is sought. Therefore, the aim of this systematic review is to collect further scientific support for the new four‐dimensional structure by specifically describing the psychosocial impact (PI) on OHRQoL in dental patients. For this purpose, dental patient populations with presumed elevated levels of PI, as well as dental patient populations with more equally distributed impacts on the four dimensions, were included in the review (ie patients with dental anxiety, oral cancer and periodontitis).
2. MATERIALS AND METHODS
The protocol that was used in this systematic review was registered in PROSPERO (CRD42017064033) and was carried out in accordance with the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) Statement.
2.1. Subjects and outcome variable of the study
The target populations selected for this review were patients with dental anxiety, oral cancer or periodontitis. These patient groups were selected, because patients with dental anxiety were assumed to be mostly affected on the psychosocial aspect of OHRQoL, relative to the other dimensions, while patients with oral cancer and patients with periodontitis were assumed to be more equally affected on the four dimensions. 12
To assess the PI of the patient group, both publications using the OHIP‐49 7 and the OHIP‐14 8 questionnaire were used. As proposed in the four‐dimensional structure for the description of OHRQoL, the handicap domain from the OHIP was used to assess patients’ PI in the present study. 12 In other words, the two items from the handicap domain of OHIP‐14 or the six items from the same domain of OHIP‐49 were used to assess the PI dimension.
2.2. Literature search
A review of the literature was conducted by a trained librarian (NTM, see Acknowledgements) who utilised natural language to identify all articles that measure OHRQoL, for any oral health condition, using the OHIP. The keywords used to retrieve the articles were ‘Oral Health Impact Profile’ or ‘OHIP’. The searches were performed in PUBMED, EMBASE, Cochrane, CINAHL and PsyINFO on 8 June 2017 and were updated in 14 January 2019. EndNote was used to remove any duplicate publications. Grey literature was not included, and authors were not contacted for additional information. For more detail on the literature search, see the methods chapter. 12
2.3. Selection criteria and study screening
Two reviewers (SS, NTM, see Acknowledgements) independently assessed the titles and abstracts of all identified studies from the electronic searches. SS and MTJ (see Acknowledgements) then determined whether an article fulfilled the criteria to be assigned to the review on the PI dimension. The inclusion and exclusion criteria for the general screening are described in the methods chapter. 12 Then, two authors (AvW and NS) of the PI dimension screened the full‐text articles based on the specific inclusion and exclusion criteria of the PI dimension presented below. In case of disagreement, a third author (CV) was added for a majority vote.
The inclusion criteria for an article to be included in the review on the PI dimension were as follows:
The OHIP‐49 or the OHIP‐14 was used for the assessment of OHRQoL;
The study sample included patients with dental anxiety, oral cancer or periodontitis;
Scores for each domain of the OHIP were reported or could be calculated;
Scores for each domain summarised using additive scores (OHIP‐ADD) method. 13 The OHIP‐ADD method is defined as the sum score of the OHIP calculated by summing the score of each item of the OHIP. The score of each item ranges from 0 to 4 and thereby the sum scores of the OHIP‐14 and the OHIP‐49 range from 0 to 56 and 0 to 196, respectively. The sum scores of the handicap domain, which was used for the PI dimension, range from 0 to 8 for the OHIP‐14 and from 0 to 24 for the OHIP‐49. Studies in which the OHIP‐ADD method was used, with the individual item scores ranging from 1 to 5, were also included and converted to match the OHIP scores as based on 0 to 4 scores.
The exclusion criteria for papers in this systematic review on the PI dimension were as follows:
Full‐text not available;
Non–English‐language articles;
2.4. Data extraction
For all included studies, the following data were extracted using a standardised form: (a) first author`s name; (b) year of publication; (c) name of the journal; (d) population of the study subjects; (e) current disease conditions of the study subjects; (f) country, (g) version of the OHIP; (h) study design (follow‐up study/cross‐sectional study); (i) proportion of male/female; (j) mean and median age with the standard deviation (SD), interquartile range (IQR), age range and 95% confidence interval (CI); (j) mean and median of the OHIP domain scores and sum scores with measures of dispersion including SD, IQR, range and 95% CI.
For included articles which had more than one patient sample, the characteristics of patients in each sample were extracted separately.
2.5. Quality assessment
The methodological quality of included studies was assessed based on a 10‐item appraisal tool for prevalence studies. 14 The risk of bias was assessed separately for each patient sample of the included studies.
Six of the 10 items were deemed useful for the risk of bias assessment of the included studies, that is representativeness of the samples, recruitment, characterisation of the subjects and the settings, coverage of the samples in the data analysis, standard criteria for measurement of the conditions and reliability of measurement of the conditions. The answer to each question is ‘Yes’, ‘No’ or ‘Unclear’. A ‘risk of bias’ judgment (‘low’, ‘unclear’ or ‘high’) was made for each signalling question. If the answer to a signalling question was judged as ‘Yes’, it was judged as ‘low risk’ of bias for this question. If the answer to a signalling question was judged as ‘No’, it was judged as ‘high risk’ of bias for this question. Otherwise, the question was judged as ‘unclear risk’. For more detail on quality assessment, see the methods chapter. 12
The quality assessment was performed independently by three authors (NS, CV and AvW), and consensus was reached through discussion.
2.6. Data synthesis and analysis
To enhance comparison of data between papers that used different versions of the OHIP, mean domain scores and 95% CI values of studies using the OHIP‐49 were converted to match mean and 95% CI of the OHIP‐14. After conversion, the mean score on each domain ranges from 0 to 8. If the mean values were not provided in the original paper, median values were used for the data analysis. If the 95% CI values were not given, they were calculated from SD values, stand error (SE) values, IQR values, or first and third quartile range values. For details, see the methods chapter. 12
For each dental disorder included in the PI dimension (dental anxiety, oral cancer and periodontitis), the pooled mean score was calculated by pooling the mean scores of the corresponding samples of patients. The pooled mean scores of each disorder were calculated weighted by the sample size of the patient populations in the included studies.
3. RESULTS
3.1. Results of search and selection
The initial search identified a total of 2104 studies. 12 After screening of the titles, abstracts and full texts, 23 studies were included in the present review (Figure 1). 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37
FIGURE 1.
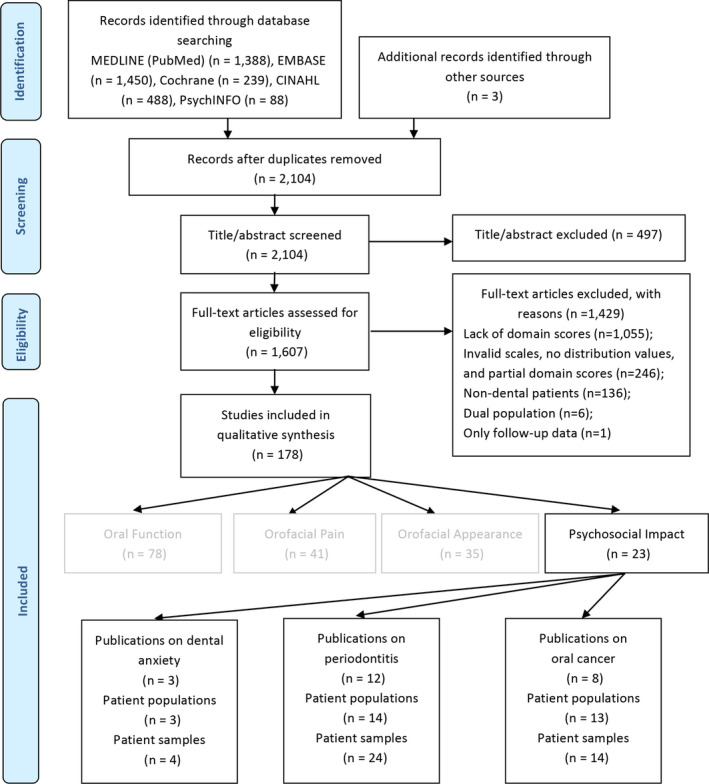
PRISMA flowchart of the inclusion process of publications in the review on the Psychosocial Impact dimension
3.2. Characteristics of included studies
The 23 included studies contained a total of 30 patient populations and 42 patient samples. 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 Among the studies, 12 studies (24 samples) involved periodontal patients, 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 8 studies (14 samples) involved oral cancer patients 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 and 3 studies (4 samples) involved anxiety patients. 15 , 16 , 17 The 23 studies were conducted in 10 countries. Most studies were performed in China (N = 5), India (N = 3), Brazil (N = 3) and Israel (N = 3). Four of the studies used the OHIP‐49 for patients’ OHRQoL while the others used the OHIP‐14. The sample characteristics are presented in more detail in Table 1.
Table 1.
OHIP scores for included patient samples in the Psychosocial Impact dimension
| Year | Population | Population, N (% women) | Mean ages (SD), Range | Instrument Original mean score (SD) | Standardised mean score (95%CI) b |
|---|---|---|---|---|---|
| Dental anxiety | |||||
| Almoznino 2015 15 |
I: Dental anxiety II: Gag reflex |
68 (36.8%) 54 (35.2%) |
OHIP‐14 4.46 (2.18) OHIP‐14 4.47 (2.25) |
4.46 (3.93‐4.99) 4.47 (3.86‐5.08) |
|
| Vermaire 2008 16 | I: Dental anxiety‐routine care | 33 (49%) |
34.1 (9.2) 18‐55 |
OHIP‐14 2.07 (1.15) |
2.07 (1.66‐2.48) |
| Vermaire 2016 17 | I: Dental anxiety | 76 (42.1%) |
42.6 (11.9) 25‐71 |
OHIP‐14 1.6 (0‐4) a |
1.6 (0.37‐2.83) |
| Oral cancer | |||||
| Barrios 2015a 18 | I: Oral cancer survivors prior to prosthetic | 142 (35.9%) | 65.2 (12.9) |
OHIP‐14 2.1 (2.1) |
2.1 (1.75‐2.45) |
| Dholam 2016 19 |
I: Prior to rehabilitation before obturator II: Prior to rehabilitation before partial denture III: Prior to rehabilitation before complete denture |
45 (42.2%) 20 (25%) 10 (10%) |
43 52 62 |
OHIP‐14 1.21 (1.27) OHIP‐14 1.10 (1.26) OHIP‐14 0.50 (0.75) |
1.21 (0.83‐1.59) 1.10 (0.51‐1.69) 0.50 (0.00‐1.03) |
| Dholam 2017 20 | I: Prior to prosthetic rehabilitation (no treatment) | 60 (31.7%) |
53 14‐73 |
OHIP‐14 0.73 (1.09) |
0.73 (0.45‐1.01) |
| Indrapriyadhar shini 2017 21 |
I: Treated by surgery and radiotherapy II: Treated by surgery alone III: Treated by surgery, chemotherapy, and radiotherapy |
30 (53.33%) 30 (76.67%) 30 (20%) |
OHIP‐14 4.64 (2.82) OHIP‐14 4.07 (2.67) OHIP‐14 5.37 (2.42) |
4.64 (3.59 5.69) 4.07 (3.07 5.07) 5.37 (4.47 6.27) |
|
| Li 2017 22 | I: Head and neck cancer, survivors | 77 (32.5%) |
67.7 (10.66) 26‐86 |
OHIP‐14 1.6 (1.6) |
1.6 (1.24 1.96) |
| McMillan 2004 23 |
I: NPC, new diagnosis II: NPC, survivors |
40 (17%) 38 (29%) |
46.7 (10.2) 50.1 (10.1) |
OHIP‐49 1.1 (0.34) OHIP‐49 3.8 (0.81) |
0.44 (0.40 0.48) 1.52 (1.41‐1.63) |
| Pow 2012 24 | I: NPC, radiation | 58 (40.6%) |
46.9 (9.5) 28‐70 |
OHIP‐49 0.9 (1.6) |
0.36 (0.19 0.53) |
| Stuani 2018 25 |
I: Head and neck cancer, prior to treatment II: Post‐radiotherapy and/or chemotherapy phase |
20 (25%) 20 (15%) |
53.75 (16.91) 58.3 (10.63) |
OHIP‐14 1.27 (1.23) OHIP‐14 1.42 (1.21) |
1.27 (0.69 1.85) 1.42 (0.85 1.99) |
| Periodontitis | |||||
| Desai 2014 26 |
I: Periodontitis: manual self‐complete II: Periodontitis: tel. interview |
OHIP‐49 0 (0‐3) a OHIP‐49 1 (0‐4) a |
0 (0.00 0.17) 0.4 (0.18 0.62) |
||
| Durham 2013 27 | I: Chronic periodontitis | 89 | 47 (9) |
OHIP‐49 2.76 (3.66) |
1.10 (0.80‐1.41) |
| Goh 2018 28 | I: Aggressive periodontitis, treated | 89 (58.4%) |
25.2 (3.2) 18‐30 |
OHIP‐14 1.1 (1.3) |
1.1 (0.83 1.37) |
| Al Habashneh 2012 29 |
I: Periodontitis, mild II: Gingivitis, chronic III: Periodontitis, moderate IV: Periodontitis, severe |
79 167 93 61 |
OHIP‐14 1.03 (1.24) OHIP‐14 1.14 (1.44) OHIP‐14 1.78 (1.66) OHIP‐14 2.11 (1.74) |
1.03 (0.75 1.31) 1.14 (0.92 1.36) 1.78 (1.44 2.12) 2.11 (1.66 2.56) |
|
| Ilanos 2018 30 |
I: Generalised aggressive periodontitis II: Generalised chronic periodontitis III: Localised aggressive periodontitis |
33 (66.6%) 10 (40%) 9 (77.7%) |
30.7 (5) 50.1 (6.8) 25.5 (7.4) |
OHIP‐14 0.8 (1.2) OHIP‐14 0.7 (1.0) OHIP‐14 0.2 (0.7) |
0.8 (0.37 1.23) 0.7 (0.00 1.42) 0.2 (0.00 0.74) |
| Jansson 2014 31 |
I: Bone loss > 1/3 root length, < 30% teeth II: Bone loss < 1/3 root length III: Bone loss > 1/3 root length,> 30% teeth |
90 (56%) 304 (52%) 49 (41%) |
59.9 (11.4) 42.5 (15.4) 64.4 (11.8) |
OHIP‐14 0.41 (1) OHIP‐14 0.37 (0.87) OHIP‐14 1.23 (1.89) |
0.41 (0.20‐0.62) 0.37 (0.27‐0.47) 1.23 (0.69‐1.77) |
| Kato 2018 32 |
I: Periodontitis, 70‐year‐old women II: Periodontitis, 70‐year‐old men III: Periodontitis, 78‐year‐old women IV: Periodontitis, 82‐year‐old women |
303 (100%) 235 (0%) 148 (100%) 118 (100%) |
70 70 78 ≥82 |
OHIP‐14 0.3 (1.0) OHIP‐14 0.3 (0.9) OHIP‐14 0.4 (1.0) OHIP‐14 0.3 (0.8) |
0.3 (0.15 0.45) 0.3 (0.18 0.42) 0.4 (0.24 0.56) 0.3 (0.19 0.41) |
| Levin 2018a 33 | I: Chronic periodontitis | 99 (18.2%) | 38.8 (7.8) |
OHIP‐14 0.69 (0.85) |
0.69 (0.55 0.83) |
| Levin 2018b 34 | I: Aggressive periodontitis | 60 | 24.7 (7.7) |
OHIP‐14 0.83 (1.03) |
0.83 (0.56 1.10) |
| Lu 2016 35 | I: Halitosis group | 102 (55.9%) | 37.7 (14.2) |
OHIP‐14 2.1 (1.7) |
2.1 (1.77 2.43) |
| Mendez 2017 36 | I: Gingivitis, moderate/severe periodontitis | 55 (65.5%) | 51.4 (9.4) |
OHIP‐14 1.4 (1.7) |
1.4 (0.94 1.86) |
| Ng 2006 37 |
II: Periodontitis, low/control I: Periodontitis, severe |
584 143 |
OHIP‐14 0.28 (0.6) OHIP‐14 0.33 (0.52) |
0.28 (0.23 0.33) 0.33 (0.24 0.42) |
|
Abbreviations: 95% CI: 95% confidence interval; NPC, Nasopharyngeal carcinoma; OHIP, Oral Health Impact Profile; SD, Standard deviation.
Median (interquartile range);
Mean scores and SD from OHIP‐49 were converted into OHIP‐14 mean and 95%CI values.
3.3. Quality assessment
Most patient samples from the studies showed high methodological quality in most categories of the tool (see Figure 2). For representativeness, 4 samples from 2 studies were regarded as having a ‘high risk’ of bias, 16 , 21 because the patient populations did not match the target patients of these studies. Four samples from another 2 studies were regarded as having ‘unclear risk’ of bias, 19 , 20 because no sufficient information on the source of included patients was provided. For recruitment, 15 samples from 8 studies were regarded as ‘high risk’ of bias, 15 , 16 , 21 , 22 , 26 , 28 , 30 , 37 because the patients’ recruitment phase was short (< 6 months), the sample size was small (<100 patients) or convenience sampling was used to recruit patients. Six samples from another 4 studies were regarded as ‘unclear risk’ of bias, 18 , 20 , 23 , 25 because of insufficient information on the type of sampling, period of time of recruitment or sample size. For characterisation, 1 sample from 1 study was regarded as ‘high risk’ of bias, 20 because the description of the characteristics of clinical settings was insufficient while no samples were regarded as ‘unclear risk’ of bias. For coverage, 4 samples from 2 studies were regarded as ‘high risk’ of bias, 28 , 31 because patients’ response rate was too low, while 31 samples from another 14 studies were regarded as ‘unclear risk’ of bias, 15 , 17 , 19 , 20 , 21 , 23 , 25 , 26 , 27 , 29 , 30 , 32 , 35 , 36 because patients’ response rate was not reported and could not be calculated. For standard, 10 samples from 5 studies were regarded as ‘high risk’ of bias, 16 , 19 , 20 , 21 , 25 because the language‐version of the OHIP used in the studies was not reported or the language‐version of the OHIP used was not validated in previous publications. No samples were regarded as having ‘unclear risk’ of bias. For reliability, 18 samples from 11 studies were regarded as ‘high risk’ of bias, 15 , 16 , 19 , 20 , 21 , 22 , 24 , 25 , 26 , 36 because the used measurement for OHRQoL in these studies was not standardised and the objective of the OHIP was not self‐reported by the patients (though with the assistance of others). No samples from the studies were regarded as ‘unclear risk’ of bias in this category (Figure 2).
FIGURE 2.
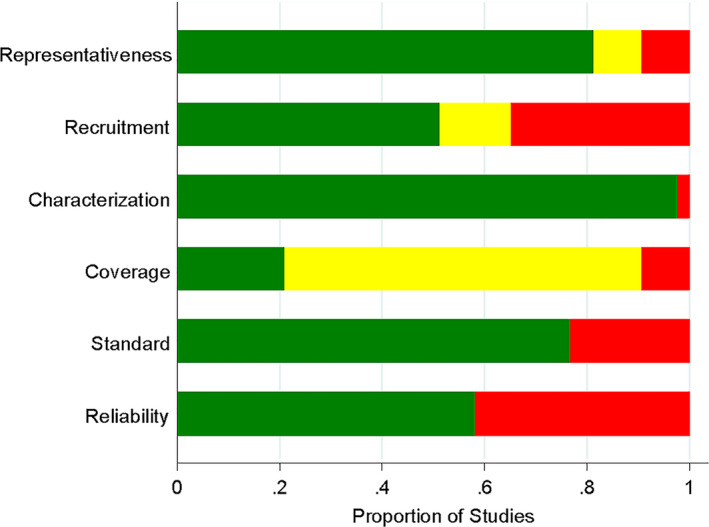
Risk of bias assessment of patient samples of included studies based on review authors` judgments about each risk of bias item presented as percentages across all included studies. Green indicates low risk of bias. Yellow indicates unclear risk of bias. Red indicates high risk of bias
3.4. Results of data synthesis and analysis
The mean scores on the PI dimension of the individual samples of patients are presented in Figure 3. It shows that patients with dental anxiety, patients with a gag reflex and patients with oral cancer treated with surgery alone, surgery and radiotherapy, or a combination of surgery, radiotherapy and chemotherapy, had significantly higher mean scores (as based on their 95%CI values) on the PI dimension as compared to the other patients samples. The mean scores for those five patient samples ranged between 4 and 5. The mean scores in the remaining samples of patients ranged between 0 and 3.
FIGURE 3.
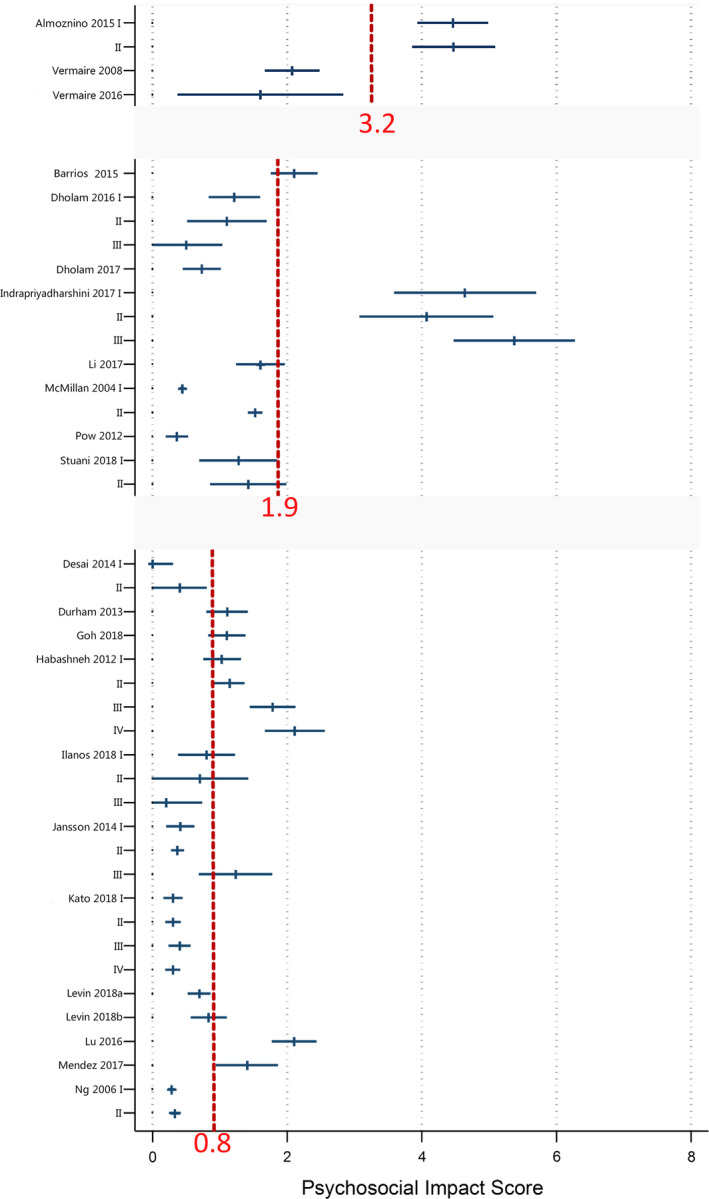
Forest plots for the mean scores of included patient samples in the Psychosocial Impact dimension based on different disease disorders. The red dotted lines represent the pooled mean scores (weighted by sample size) of each disease disorder
The pooled mean score for dental anxiety patient samples (ie 3.2) was the highest, while the pooled mean score for periodontitis patient samples (ie 0.8) was the lowest. The pooled mean score for oral cancer patient samples was 1.9. This suggests that patients with dental anxiety perceive the strongest effect on the PI dimension, while patients with periodontitis perceive the weakest effect on the PI dimension, among these three types of dental disorders.
4. DISCUSSION
The aim of the present systematic review was to collect further scientific support for the new four‐dimensional structure of OHRQoL by specifically describing the PI dimension in the three dental patient populations. The samples consisted of patients with dental anxiety, oral cancer or periodontitis. A total of 23 studies were included, which covered 42 samples of patients, including 24 samples of periodontal patients, 14 samples of cancer patients and 4 samples of dentally anxious patients. Regarding the quality of the included studies, the majority showed high methodological quality on 3 specific categories (representativeness, characterisation and standard). On 2 categories (recruitment and reliability) a modest quality was observed, while the lowest quality was scored on the category coverage, with a large number of the samples rated as ‘unclear’. All in all, this suggests that the included studies were of adequate methodological quality. The present review provides standardised information regarding three dental patient populations that can be used as reference for the PI dimension within the newly proposed OHRQoL four‐dimensional structure.
Results showed that the pooled mean scores on the PI domain were relatively low for the sample of periodontitis (0.8) and oral cancer patients (1.9). In comparison, the sample of dentally anxious patients scored significantly higher (3.2). In other words, patients with dental anxiety perceived a stronger impact on the PI dimension of their OHRQoL than those with periodontitis or oral cancer. One explanation for this surprising outcome (especially for the relatively low score of the patients with oral cancer) resembles something referred to as the ‘disability paradox’. This paradox refers to the observation that many people with serious and persistent disabilities report that they experience a good or excellent quality of life while to most external observers these individuals seem to live an undesirable daily existence. 38 Something similar might hold true for the oral cancer patients. For instance, some of these patients require invasive surgery, which can result in severe aesthetic impairment and a plausible subsequent impact on their psychosocial wellbeing (social isolation, feelings of shame, depressed, being stared at, etc). On the other hand, oral cancer patients also face a potentially life‐threatening condition which can put things into a different perspective. Especially when facing or having faced death, people tend to reconsider what they value as important, which is often family, friends and loved ones. So, contrary to the ‘plausible impact’ just mentioned, oral cancer patients may actually experience an increase in the quality of their social relations since these are valued higher. Social interactions may also be intensified given the life‐threatening condition these patients suffer from. Moreover, having survived cancer can give new meaning to live. Any possible aesthetic impairment as mentioned above can therefore seem relatively less important than before.
Periodontal problems, in the broadest sense of the word, were expected to impact the psychosocial dimension in a different way. For instance, the PI could be elevated because of possible impaired aesthetics due to missing teeth, or bad breath, perhaps hindering patients in their interaction with others. The results, however, do not support a strong impact on the PI dimension for patients with periodontitis. In fact, the category of periodontal patients, which contained the highest number of patients and samples, shows a consistent result regarding a relatively low mean score on the psychosocial domain.
As illustrated in Figure 3, large heterogeneity was found across the studies included in each of the dental disorder groups. One of the reasons for the large heterogeneity may be that individuals` self‐perception of their psychosocial wellbeing is a relatively subjective and unstable experience, which could be impacted by cultural norms of a society. 39 Culture influences the way individuals think about, express and define their worlds. 40 Individuals` perception of quality of life therefore depends on the cultural context and values of the system in which they live, and is associated with their goals, expectations, standards and concerns. 41 Therefore, patients from different cultures may score differently on the PI dimension for equally serious disorders. Another reason for the large heterogeneity is that the included patients may differ in the severity of the disorder. For example, in the paper by Al Habashneh et al, 29 patients with severe periodontitis scored twice as high on the PI dimension as compared to the patients with mild periodontitis.
One limitation of the present review concerns the fact that not all available studies could be included. For example, one study used a unique scoring system in which the domains of OHIP‐14 were scored from 0 to 100. 42 Another study 43 did not use the OHIP‐ADD method to calculate the sum score of the OHIP‐14. Instead, the OHIP simple counting (OHIP‐SC) scoring method was used. The OHIP‐SC is defined as the sum score of the OHIP calculated by the number of the items of the OHIP with impacts reported at a threshold level (for example, ‘fairly often’ or ‘very often’). 44 Therefore, these data were not suitable for synthesis with results from the articles included in the present study. For more detailed information regarding the measurement of OHRQoL and its related practical issues please see the chapter by Reissman. 45
In addition, only studies published in English were eligible to be included, and the grey literature was not investigated. Also, even though multiple versions of the OHIP exist, only the OHIP‐14 and the OHIP‐49 were included, while other versions of the OHIP, for instance, OHIP‐EDENT which was tailored for patients with prosthetics or edentulous patients, 46 were excluded. It is complex to argue which specific effect these limitations may have had on the outcomes of this review.
Another limitation is related to grouping of different types of patients into one dental patient category. Consider for instance the periodontal patients, a category that contains patients with many different types of problems such as halitosis, (chronic) gingivitis, (chronic/aggressive) periodontitis or (mild/moderate/severe) chronic periodontitis. Another example relates to the oral cancer patients, where some patient samples included patients with a recent diagnosis of cancer prior to treatment, while other samples represented cancer survivors who had been treated. This begs the question whether or not it makes sense, and whether it is justified, to put different subtypes of patients within one overall category. However, if such heterogeneity exists between groups of patients in the same category, it is still possible to look at the studies separately, since the estimates of the PI dimension are reported for each included individual study as displayed in Figure 3 and Table 1.
The 95% CIs of the mean scores for the three dental disorders were not presented. Therefore, even though it was visualised that the patients with dental anxiety had the highest pooled mean score and the patients with periodontitis had the lowest pooled mean score, it was impossible to report whether the difference was statistically significant. This is because the 95% CI values of individual patient samples on a 0 to 8 metric converted from the OHIP‐49 may have less range than the 95% CI values of the same patient samples from the OHIP‐14 due to the method we used. Therefore, we think the 95% Cl values of individual patient samples from the OHIP‐14 are too heterogeneous to be meaningfully combined with the converted 95% CI values from the OHIP‐49. This is another limitation of the study.
Further, only patients with one of the three types of dental disorders (dental anxiety, periodontitis and oral cancer) were included in the present study. Patients with other dental disorders, for example, temporomandibular disorders (TMDs) or edentulous patients, may be more likely to have psychosocial disorders than those with periodontitis. However, those patients were not included in the current study because it was pre‐assumed that they would be affected more by items from one of the other dimensions. Therefore, studies on TMD patients were assigned to the review on the Orofacial Pain dimension and studies on edentulous patients were assigned to the review on the Oral Function dimension. This is another limitation of the present review. Future studies are recommended to focus on the PI of the patients with broader dental disorders, such as TMDs and edentulism.
5. CONCLUSION
In summary, within its limitations, the present study provides standardised information about the PI dimension, within the proposed four‐dimensional model of OHRQoL, for three dental patient groups (dental anxiety, periodontitis and oral cancer). Results indicate that dental anxiety may have the strongest effect on the PI dimension, while periodontitis may have the weakest effect on the PI dimension among the three dental disorders. Future studies need to confirm whether the reported differences on the PI dimension between the three dental disorders are statistically significant and clinically relevant.
CONFLICT OF INTEREST
The authors report no conflict of interest.
AUTHOR CONTRIBUTIONS
NS contributed to concept, study design, full‐text assessment, risk of bias assessment and interpretation of results. AvW and CM contributed to concept, study design, full‐text assessment and risk of bias assessment. All authors critically revised the manuscript and provided final approval before submission.
Peer Review
The peer review history for this article is available at https://publons.com/publon/10.1111/joor.13064.
ACKNOWLEDGMENT
We gratefully acknowledged Stella Sekulic, Mike T. John and Nicole Theis‐Mahon for their assistance in the electronic literature searching, screening of abstracts and assessment of full‐text articles.
Su N, Wijk A, Visscher CM. Psychosocial oral health‐related quality of life impact: A systematic review. J Oral Rehabil.2021;48:282–292. 10.1111/joor.13064
REFERENCES
- 1. Visscher CM, Baad‐Hansen L, Durham J, et al. Benefits of implementing pain‐related disability and psychological assessment in dental practice for patients with temporomandibular pain and other oral health conditions. J American Dental Assoc. 2018;149:422‐431. [DOI] [PubMed] [Google Scholar]
- 2. Gatchel RJ, Okifuji A. Evidence‐based scientific data documenting the treatment and cost‐effectiveness ofcomprehensive pain programs for chronic nonmalignant pain. J Pain. 2006;7:779‐793. [DOI] [PubMed] [Google Scholar]
- 3. Schiffman E, Ohrbach R, Truelove E, et al. Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) for clinical and research applications: recommendations of the International RDC/TMD Consortium Network and Orofacial Pain Special Interest Group. J Oral Facial Pain Headache. 2014;28:6‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kroenke K, Spitzer RL, Williams JB, Löwe B. An ultra‐brief screening scale for anxiety and depression: the PHQ‐4. Psychosomatics. 2009;50:613‐621. [DOI] [PubMed] [Google Scholar]
- 5. Löwe B, Wahl I, Rose M, et al. A 4‐item measure of depression and anxiety: validation and standardization of the Patient Health Questionnaire‐4 (PHQ‐4) in the general population. J Affect Disord. 2010;122:86‐95. [DOI] [PubMed] [Google Scholar]
- 6. Van Der Lugt CM, Rollman A, Naeije M, Lobbezoo F, Visscher CM. Social support in chronic pain: development and preliminary psychometric assessment of a new instrument. J Oral Rehabil. 2012;39:270‐276. [DOI] [PubMed] [Google Scholar]
- 7. Slade GD, Spencer AJ. Development and evaluation of the Oral Health Impact Profile. Community Dent Health. 1994;11:3‐11. [PubMed] [Google Scholar]
- 8. Slade GD. Derivation and validation of a short‐form oral health impact profile. Community Dent Oral Epidemiol. 1997;25:284‐290. [DOI] [PubMed] [Google Scholar]
- 9. Waller N, John MT, Feuerstahler L, et al. A 7‐day recall period for a clinical application of the oral health impact profile questionnaire. Clin Oral Investig. 2016;20:91‐99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. John MT, Reissmann DR, Čelebić A, et al. Integration of oral health‐related quality of life instruments. J Dent. 2016;53:38‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mittal H, John MT, Sekulić S, Theis‐Mahon N, Rener‐Sitar K. Patient‐reported outcome measures for adult dental patients: a systematic review. J Evid Based Pract. 2019;19:53‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sekulic S, John MT, Häggman‐Henrikson B, Theis‐Mahon N. Dental patients` functional, pain‐related, aesthetic, and psychosocial impact of oral conditions on quality of life‐Project overview, data collection, quality assessment, and publication bias. J Oral Rehabil. 2020. 10.1111/joor.13045. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Slade GD, Strauss RP, Atchison KA, Kressin NR, Locker D, Reisine ST. Conference summary: assessing oral health outcomes – measuring health status and quality of life. Community Dent Health. 1998;15:3‐7. [PubMed] [Google Scholar]
- 14. Munn Z, Moola S, Riitano D, Lisy K. The development of a critical appraisal tool for use in systematic reviews addressing questions of prevalence. Int J Health Policy Manag. 2014;3:123‐128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Almoznino G, Zini A, Aframian DJ, et al. Oral health related quality of life in young individuals with dental anxiety and exaggerated gag reflex. Oral Health Prev Dent. 2015;13:435‐440. [DOI] [PubMed] [Google Scholar]
- 16. Vermaire JH, de Jongh A, Aartman IH. Dental anxiety and quality of life: the effect of dental treatment. Community Dent Oral Epidemiol. 2008;36:409‐416. [DOI] [PubMed] [Google Scholar]
- 17. Vermaire JH, van Houtem CM, Ross JN, Schuller AA. The burden of disease of dental anxiety: generic and disease‐specific quality of life in patients with and without extreme levels of dental anxiety. Eur J Oral Sci. 2016;124:454‐458. [DOI] [PubMed] [Google Scholar]
- 18. Barrios R, Bravo M, Gil‐Montoya JA, Martínez‐Lara I, García‐Medina B, Tsakos G. Oral and general health‐related quality of life in patients treated for oral cancer compared to control group. Health Qual Life Outcomes. 2015;13:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dholam KP, Chouksey GC, Dugad J. Oral health‐related quality of life after prosthetic rehabilitation in patients with oral cancer: a longitudinal study with the Liverpool Oral Rehabilitation Questionnaire version 3 and Oral Health Impact Profile‐14 questionnaire. Indian J Cancer. 2016;53:256‐260. [DOI] [PubMed] [Google Scholar]
- 20. Dholam KP, Dugad JA, Sadashiva KM. Impact of oral rehabilitation on patients with head and neck cancer: a study using the Liverpool Oral Rehabilitation Questionnaire and the Oral Health Impact Profile‐14. J Prosthet Dent. 2017;117:559‐562. [DOI] [PubMed] [Google Scholar]
- 21. Indrapriyadharshini K, Madankumar PD, Karthikeyan GR. Oral health‐related quality of life in patients treated for oral malignancy at Kanchipuram district, India: a cross‐sectional study. Indian J Cancer. 2017;54:11‐15. [DOI] [PubMed] [Google Scholar]
- 22. Li N, Otomaru T, Taniguchi H. Sleep quality in long‐term survivors of head and neck cancer: preliminary findings. Support Care Cancer. 2017;25:3741‐3748. [DOI] [PubMed] [Google Scholar]
- 23. McMillan AS, Pow EH, Leung WK, Wong MC, Kwong DL. Oral health‐related quality of life in southern Chinese following radiotherapy for nasopharyngeal carcinoma. J Oral Rehabil. 2004;31:600‐608. [DOI] [PubMed] [Google Scholar]
- 24. Pow EH, Kwong DL, Sham JS, Lee VH, Ng SC. Can intensity‐modulated radiotherapy preserve oral health‐related quality of life of nasopharyngeal carcinoma patients? Int J Radiat Oncol Biol Phys. 2012;83:e213‐e221. [DOI] [PubMed] [Google Scholar]
- 25. Stuani VT, Santos PSS, Damante CA, et al. Oral health impact profile of head and neck cancer patients after or before oncologic treatment: an observational analytic case‐control study. Support Care Cancer. 2018;26:2185‐2189. [DOI] [PubMed] [Google Scholar]
- 26. Desai R, Durham J, Wassell RW, Preshaw PM. Does the mode of administration of the Oral Health Impact Profile‐49 affect the outcome score? J Dent. 2014;42:84‐89. [DOI] [PubMed] [Google Scholar]
- 27. Durham J, Fraser HM, McCracken GI, Stone KM, John MT, Preshaw PM. Impact of periodontitis on oral health‐related quality of life. J Dent. 2013;41:370‐376. [DOI] [PubMed] [Google Scholar]
- 28. Goh V, Nihalani D, Yeung KWS, Corbet EF, Leung WK. Moderate‐ to long‐term therapeutic outcomes of treated aggressive periodontitis patients without regular supportive care. J Periodontal Res. 2018;53:324‐333. [DOI] [PubMed] [Google Scholar]
- 29. Al Habashneh R, Khader YS, Salameh S. Use of the Arabic version of Oral Health Impact Profile‐14 to evaluate the impact of periodontal disease on oral health‐related quality of life among Jordanian adults. J Oral Sci. 2012;54:113‐120. [DOI] [PubMed] [Google Scholar]
- 30. Llanos AH, Silva CGB, Ichimura KT, et al. Impact of aggressive periodontitis and chronic periodontitis on oral health‐related quality of life. Braz Oral Res. 2018;32:e006. [DOI] [PubMed] [Google Scholar]
- 31. Jansson H, Wahlin Å, Johansson V, et al. Impact of periodontal disease experience on oral health‐related quality of life. J Periodontol. 2014;85:438‐445. [DOI] [PubMed] [Google Scholar]
- 32. Kato T, Abrahamsson I, Wide U, Hakeberg M. Periodontal disease among older people and its impact on oral health‐related quality of life. Gerodontology. 2018;35:382‐390. [DOI] [PubMed] [Google Scholar]
- 33. Levin L, Zini A, Levine J, et al. Demographic profile, Oral Health Impact Profile and Dental Anxiety Scale in patients with chronic periodontitis: a case‐control study. Int Dent J. 2018;68:269‐278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Levin L, Zini A, Levine J, et al. Dental anxiety and oral health‐related quality of life in aggressive periodontitis patients. Clin Oral Investig. 2018;22:1411‐1422. [DOI] [PubMed] [Google Scholar]
- 35. Lu HX, Chen XL, Wong M, Zhu C, Ye W. Oral health impact of halitosis in Chinese adults. Int J Dent Hyg. 2017;15:e85‐e92. [DOI] [PubMed] [Google Scholar]
- 36. Mendez M, Melchiors Angst PD, Stadler AF, Oppermann RV, Gomes S. Impacts of supragingival and subgingival periodontal treatments on oral health‐related quality of life. Int J Dent Hyg. 2017;15:135‐141. [DOI] [PubMed] [Google Scholar]
- 37. Ng SK, Leung WK. Oral health‐related quality of life and periodontal status. Community Dent Oral Epidemiol. 2006;34:114‐122. [DOI] [PubMed] [Google Scholar]
- 38. Albrecht GL, Devlieger PJ. The disability paradox: high quality of life against all odds. Soc Sci Med. 1999;48:977‐988. [DOI] [PubMed] [Google Scholar]
- 39. Lehman DR, Chiu C, Schaller M. Psychology and culture. Annu Rev Psychol. 2004;55:689‐714. [DOI] [PubMed] [Google Scholar]
- 40. Noguchi K, Kamada A, Shrira I. Cultural differences in the primary effect for person perception. Int J Psychol. 2014;49:208‐210. [DOI] [PubMed] [Google Scholar]
- 41. Harper A. Development of the World Health Organization WHOQOL‐BREF quality of life assessment. Psychol Med. 1998;28:551‐558. [DOI] [PubMed] [Google Scholar]
- 42. Li W, Zhang P, Li R, Liu Y, Kan Q. Radial free forearm flap versus pectoralis major pedicled flap for reconstruction in patients with tongue cancer: Assessment of quality of life. Med Oral Patol Oral Cir Bucal. 2016;21:e737‐e742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Barrios R, Tsakos G, García‐Medina B, Martínez‐Lara I, Bravo M. Oral health‐related quality of life and malnutrition in patients treated for oral cancer. Support Care Cancer. 2014;22:2927‐2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Slade GD. Measuring Oral Health and Quality of Life. Chapel Hill, NC: University of North Carolina; 1997:96. [Google Scholar]
- 45. Reissmann DR. Methodological considerations when measuring oral health‐related quality of life. J Oral Rehabil. 2020. 10.1111/joor.12983. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 46. Schimmel M, Leemann B, Christou P, et al. Oral health‐related quality of life in hospitalised stroke patients. Gerodontology. 2011;28:3‐11. [DOI] [PubMed] [Google Scholar]


