Figure 4. Differential regulation of the FGF14:FGF14 dimer and FGF14:Nav1.6 complex by JAK2, but not Src.
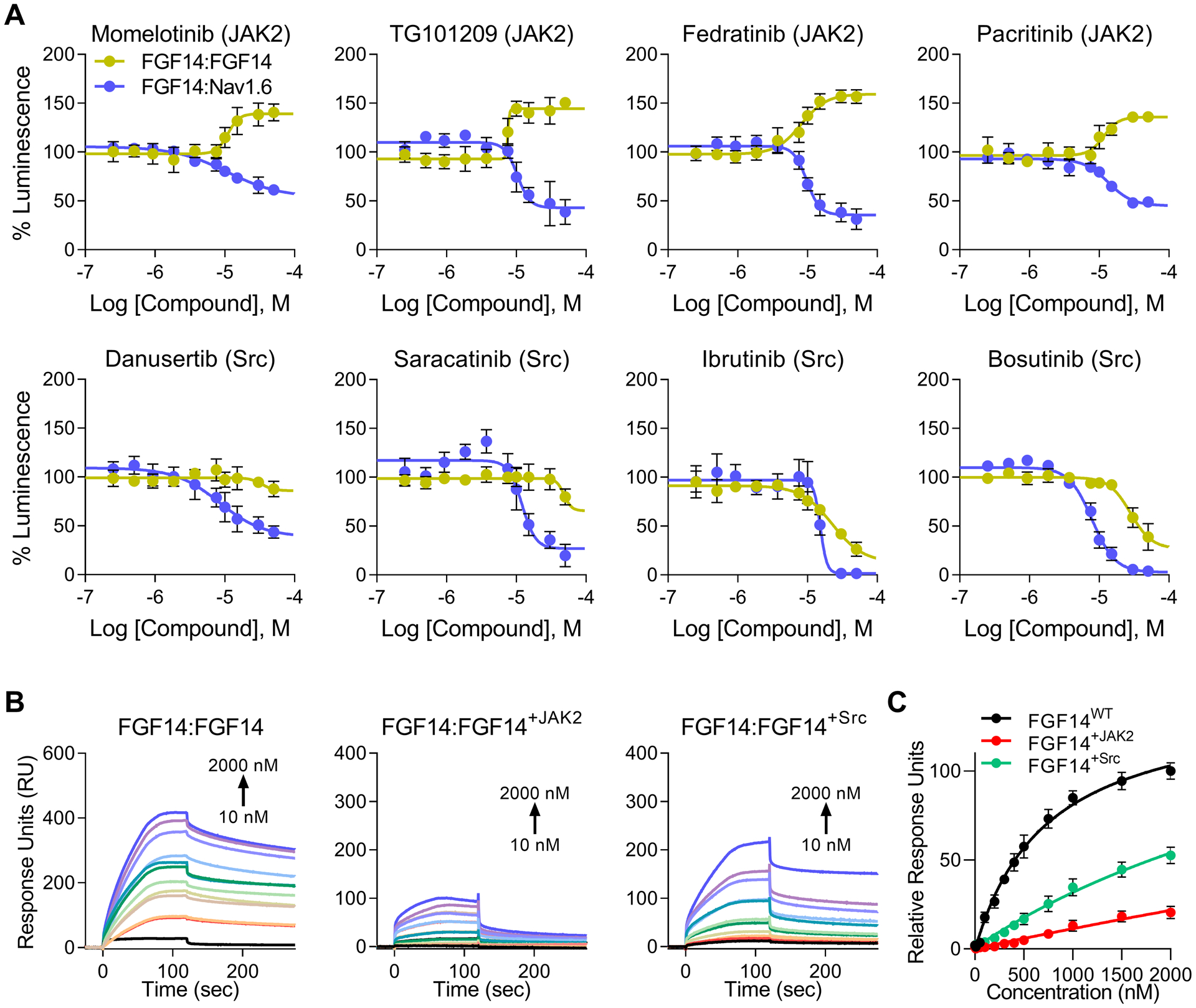
(A) Dose responses (10-point, n = 8 per concentration over two 384-well plates) were conducted against the FGF14:Nav1.6 complex (purple) for promising hits using repurchased compounds in order to validate HTS results. Positive hits were then counter-screened against the FGF14:FGF14 dimer (yellow), with the hypothesis that changes in FGF14 dimerization could be associated with inverse changes in FGF14:Nav1.6 binding. Inhibition of JAK2 but not Src, increases FGF14 dimerization in a manner directly inverse to FGF14:Nav1.6 complex formation. Estimated efficacy and potency are shown in Table 3. Luminescence for each well was normalized to per plate 0.3% DMSO controls (n = 32 per plate), and the mean normalized luminescence ± SD is shown. (B) Representative SPR sensorgrams from proteins flown across a chip with FGF14 bound (1,030 RU) using a flow rate of 50 μL/min. Purified FGF14 protein was phosphorylated in vitro by pre-incubation with either JAK2 or Src tyrosine kinases as indicated above each panel. The resulting equilibrium dissociation constants (KD), as well as kinetic association (kon) and dissociation (koff) rates are provided in Table 4. (C) Steady-state saturation plot for comparison of wild-type (WT) versus phosphorylated protein binding to FGF14 with response units (RU) relative to the maximal binding response of the WT protein. Data are mean normalized response units ± SD.
