Figure 5. MALDI TOF-MS/MS validation of JAK2 phosphorylation of Y158 on FGF14.
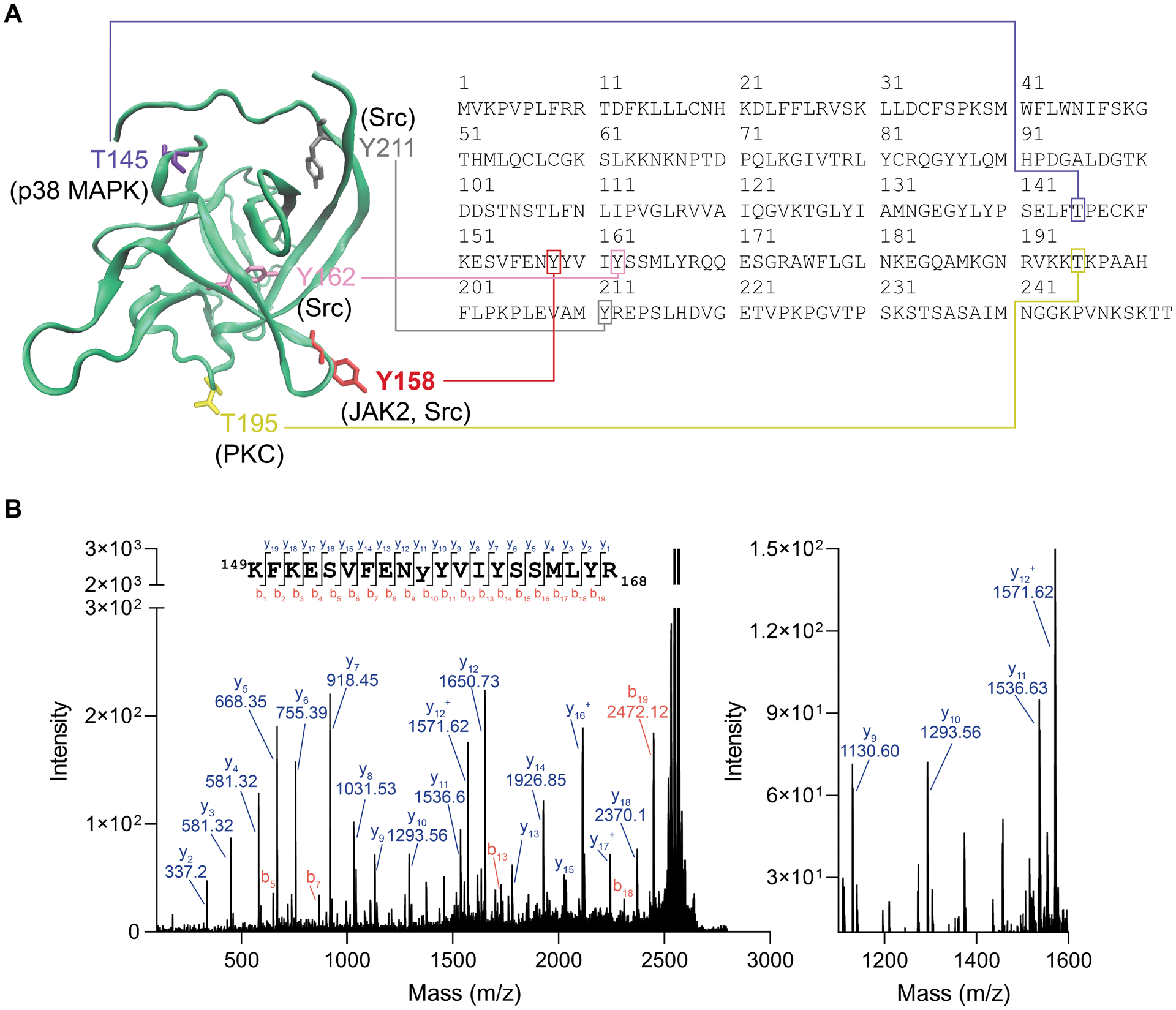
(A) Homology model of an FGF14 monomer showing potential phosphorylation sites and their corresponding motif in the FGF14–1b sequence (accession number NP_787125). Y158, red, while Y162 is shown as purple. Also showing other predicted phosphorylation sites that are not at the protein:protein interaction interface, including T145, T195, and Y211. (B) MALDI TOF-MS/MS fragmentation spectrum of the phosphopeptide KFKESVFENyYVIYSSMLYR (y = phosphotyrosine), encompassing residues 149–168 of FGF14–1b. The presence of y10 (theoretical m/z of 1293.66, observed m/z of 1293.56) and y11 (theoretical m/z of 1536.69, observed m/z of 1536.63) ions confirms Y158 as the site of phosphorylation (1536.63–1293.56 = 243.07, corresponding to the MW of Y(PO3).
