Figure 6. Y158 mediates both JAK2 regulation of FGF14, as well as high affinity dimerization.
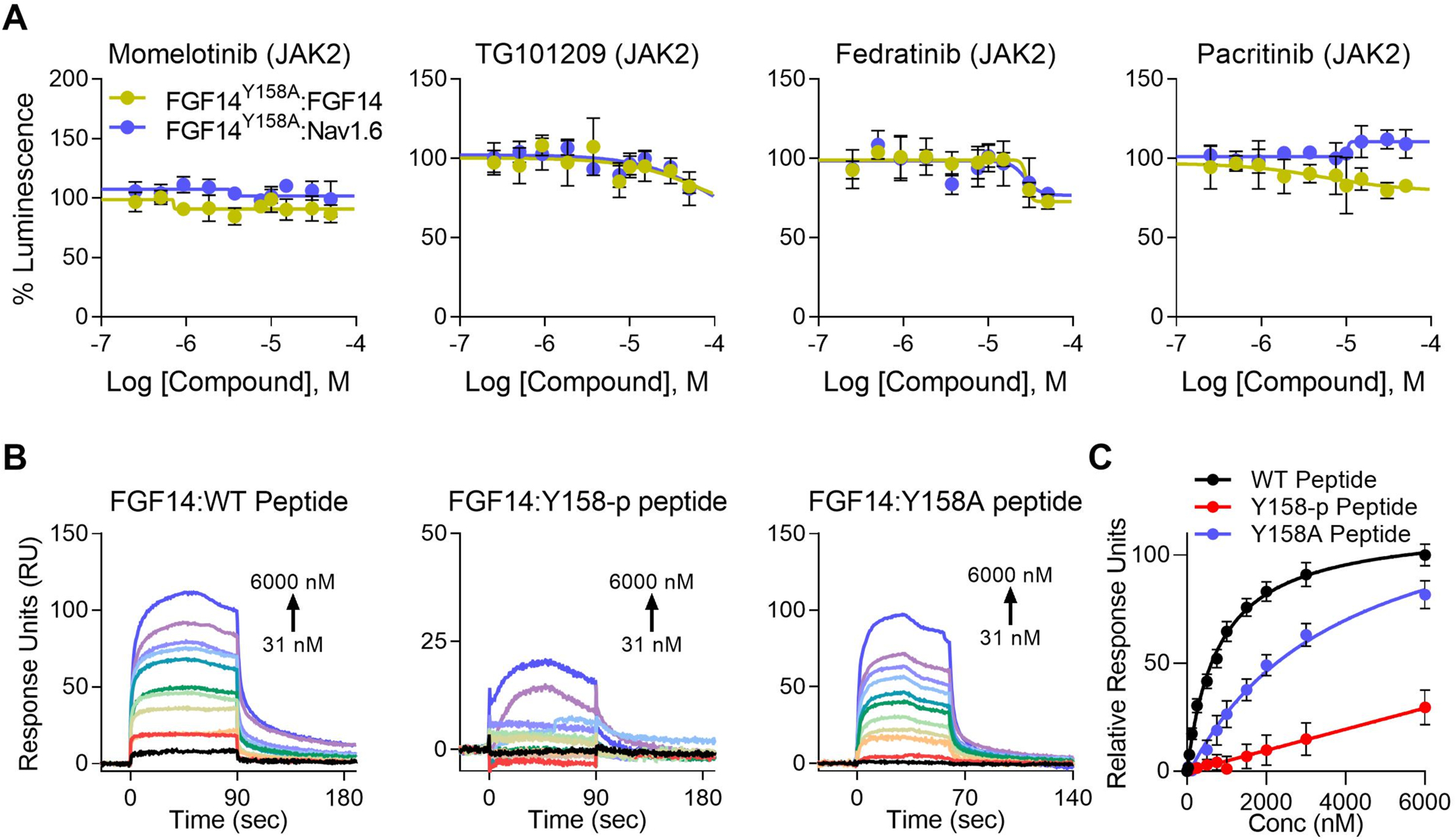
(A) Dose responses (10-point, n = 8 per concentration over two 384-well plates). Differential regulation of JAK2 inhibitors between the FGF14:Nav1.6 complex and FGF14:FGF14 dimer is almost completely abolished when tested against FGF14Y158A:Nav1.6 and FGF14Y158A:FGF14 mutant complexes using LCA. Furthermore, the effect of Fedratinib was reversed for the FGF14Y158A:FGF14 heterodimer, with high concentrations mildly decreasing dimerization. Estimated efficacy and potency are shown in Table 3. Data are mean normalized luminescence ± SD. (B) Representative SPR sensorgrams showing increasing concentrations of non-phosphorylated wild-type FGF14 peptide (WT peptide, left), FGF14 peptide phosphorylated at Y158 (Y158-p peptide, middle), or FGF14Y158A mutant peptide (Y158A peptide, right) flown over recombinant FGF14 protein bound to CM5 chips (16,045 RU) using a flow rate of 60 μL/min and concentrations ranging from 31 – 6000 nM. Phosphorylation (or lack thereof) at Y158 was verified by mass spectrometry (Figure 5). Kinetic analysis of each ligand:analyte interaction was obtained by fitting the response data to the simplest Langmuir 1:1 interaction model (KD=koff/kon). The resulting equilibrium dissociation constants (KD), as well as kinetic association (kon) and dissociation (koff) rates are provided in Table 5. The phosphorylated FGF14Y158-p peptide has reduced binding affinity for FGF14. (C) Steady-state saturation plot for comparison of wild-type (WT, black) versus phosphorylated (Y158-p, orange) and mutant (Y158A) peptide binding (black, WT; orange, phosphorylated (Y158-p); teal, Y158A mutant) to FGF14 with response units (RU) relative to the maximal binding response of the WT peptide. Data are mean normalized response units ± SD.
