FIGURE 3.
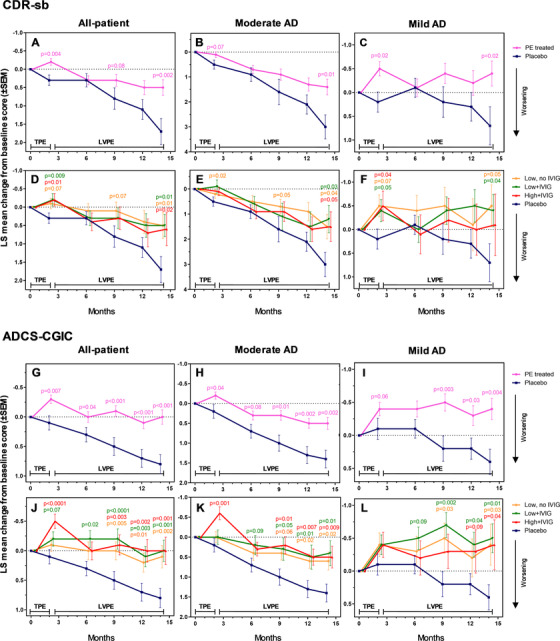
Least square (LS) mean change from baseline scores (± standard error of the mean) in the Clinical Dementia Rating Sum of Boxes (CDR‐sb), and Alzheimer's Disease Cooperative Study‐Clinical Global Impression of Change (ADCS‐CGIC) scales (global efficacy secondary variables; panels A–F and G–L, respectively) performed on mild to moderate Alzheimer's disease (AD) patients (all‐patient [panels A, D, G, J], moderate AD [panels B, E, H, K], and mild AD [panels C, F, I, L] populations) treated with plasma exchange (PE) with albumin replacement. TPE denotes the 2‐month period of conventional therapeutic PE; LVPE denotes the period up to month 14 of low‐volume PE. The difference between the treated patient groups (PE‐treated patients combined [n = 242; panels A–C and G–I], and three active groups: low/high‐albumin dose, with/without intravenous immunoglobulin [n = 78–86; panels D–F and J–L]) and the placebo group (n = 80) at months 2, 6, 9, 12, and 14 was evaluated using analysis of covariance with treatment group as a fixed effect, and the corresponding baseline value, age and AD severity, as a covariate. Both statistical significance (P < .05) and borderline significance (P < .1) versus placebo are indicated
