Abstract
BACKGROUND AND PURPOSE:
In the past decades, a large body of work aimed at investigating brain structural anomalies accrued in autism spectrum disorder. Autism spectrum disorder is associated with intellectual disability in up to 50% of cases. However, only a few neuroimaging studies were conducted in autism spectrum disorder with intellectual disability, and none of them benefited from a nonsyndromic intellectual disability control group.
MATERIALS AND METHODS:
We performed a voxelwise investigation of the structural alterations in 25 children with autism spectrum disorder with intellectual disability by comparing them with 25 typically developing children and 25 nonsyndromic children with an intellectual disability. Besides a classic voxel-based morphometry statistical approach, the threshold-free cluster enhancement statistical approach was adopted.
RESULTS:
Classic voxel-based morphometry results did not survive family-wise error correction. The threshold-free cluster enhancement–based analysis corrected for family-wise error highlighted the following: 1) widespread focal cortical anomalies and corpus callosum alteration detected in autism spectrum disorder with intellectual disability; 2) basal ganglia and basal forebrain alteration detected both in autism spectrum disorder with intellectual disability and in nonsyndromic intellectual disability; and 3) differences in the frontocingulate-parietal cortex between autism spectrum disorder with intellectual disability and nonsyndromic intellectual disability.
CONCLUSIONS:
The present study suggests that the frontocingulate-parietal cortex may be the eligible key region for further investigations aiming at detecting imaging biomarkers in autism spectrum disorder with intellectual disability. The detection of structural alterations in neurodevelopmental disorders may be dramatically improved by using a threshold-free cluster enhancement statistical approach.
The autism spectrum disorder (ASD) is a life-long neurodevelopmental disorder, the underlying biologic causes of which remain to be established. The ASD includes diverse endophenotypes sharing 2 clusters of core symptoms, such as persistent deficits in social communication and social interaction across multiple contexts and restricted, repetitive patterns of behaviors, interests, and activities.1
In past decades, a large body of work has accrued investigating anomalies in ASD by using neuroimaging techniques. Most of the studies were performed in normally gifted (not affected by mental retardation) patients with ASD or Asperger syndrome. However, a large proportion of patients with ASD are intellectually impaired. The patients with ASD with an intelligence quotient (IQ) lower than 70 are usually referred to as having low-functioning ASD or ASD with intellectual disability (ID). Epidemiologic surveys of ASD reported up to a 50% prevalence of ASD with ID, with a degree of variation due to differences in diagnostic criteria and difficulty in assessing IQ in patients with ASD as well as genetic and environmental variables.2,3 Despite the high prevalence of ASD with ID, these patients were rarely examined, probably because of the difficulties in cooperation for neuroradiologic investigations.
Only a few studies performed voxelwise investigation of structural anomalies in ASD with ID,4–7 and none of them benefited from a nonsyndromic ID control group.
In past years, advanced nonparametric statistical approaches were developed in neuroimaging. Among these, threshold-free cluster enhancement (TFCE) aims to address some well-known voxel-based morphometry (VBM) criticalities, such as smoothing kernel extent, threshold dependence, and localization of the results. TFCE was shown to improve the detection of structural anomalies in neurodegenerative disease and psychiatric disorder studies.8–10 However, the nature of structural anomalies in neurodevelopmental disorders may differ from those in neurodegenerative diseases and psychiatric disorders, and detection improvements in such disorders have not yet been evaluated.
According to our hypotheses, ASD with ID may show structural differences from both typically developing (TD) children and nonsyndromic ID that can be better detected by TFCE-VBM. The present study had the following aims: 1) to detect structural anomalies in the under-researched condition of ASD with ID, also directly comparing those patients with subjects with nonsyndromic ID; and 2) to verify the improvement in detecting structural anomalies in neurodevelopmental disorders by using TFCE in comparison with the classic VBM approach.
Materials and Methods
Participants
Twenty-five subjects with idiopathic ASD with ID (22 males, 3 females; age range, 2.4–12.7 years; mean, 6.11 ± 3.10 years) and 25 age-matched children with nonsyndromic ID (16 males, 9 females; age range, 2.1–12.4 years; mean, 7 ± 3.1 years) were recruited from patients attending the Developmental Neurology Unit from January 2008 to January 2012. All patients were evaluated by a pediatric neurologist, a clinical geneticist, and a child neuropsychologist. The patients with ASD were diagnosed in accordance with the Diagnostic and Statistical Manual of Mental Disorders-DSM-5 criteria and confirmed by the Autism Diagnostic Observation Schedule-Generic11 and the Autism Diagnostic Interview-Revised.12
Twenty-five age-matched TD children (12 males, 13 females; age range, 2.1–12.3 years; mean, 6.11 ± 2.6 years) were also enrolled. The TD children were recruited among the children of the staff involved in the study and among inpatients with suspected spinal cord abnormalities whose brain and spine examination findings were normal.
Cognitive functioning was assessed by using the Wechsler Intelligence Scales,13,14 according to their age, and the Griffiths Mental Developmental Scale15,16 for children with a chronologic or mental age younger than 4 years, considering the correlation between the Wechsler Preschool Scale Full IQ and the General Quotient obtained by the Griffiths Scales.17
The parents of ID and TD children were asked to fill out the lifetime version of the Social Communication Questionnaire,18 a self-report questionnaire that provides valuable information on a child's body movements, use of language or gestures, and style of interacting, through 40 questions based on the Autism Diagnostic Interview-Revised items. It is not meant to provide a detailed diagnosis of ASD but to indicate whether a child needs a more careful and in-depth evaluation. The cutoff score is 15.
The exclusion criteria were as follows: 1) preterm birth or known pregnancy complications or perinatal injury history; 2) associated seizures or neurologic diseases, known infectious, metabolic, or genetic diseases (high-resolution karyotype, DNA analysis of fragile-X syndrome); 3) the presence of major cerebral malformations (details in Erbetta et al19); 4) anomalies of the visceral organs and/or facial dysmorphisms; 5) Social Communication Questionnaire score higher than the cutoff of 15 for children with ID and TD; 6) the presence of severe ID because the clinical differential diagnosis between ASD and severe ID is arguable and the administration of autism diagnostic tools is not recommended in such patients.
All patients and TD children younger than 6 years of age were examined under propofol sedation (1 mg/Kg).
All the examinations were performed with written informed consent of the subject's parents. The study was approved by the Ethics Committee of Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Neurologico C. Besta.
Data Acquisition
Volumetric T1-weighted images were acquired on a 1.5T MR imaging system (Magnetom Avanto; Siemens, Erlangen, Germany) by using a magnetization-prepared rapid acquisition of gradient echo sequence (TR = 1640 ms, TE = 2.48 ms, TI = 552 ms, FOV = 256 × 256 mm, matrix = 256 × 256, 160 sagittal sections, voxel size = 1 × 1 × 1 mm). Structural imaging included axial proton-density/T2-weighted images (TR = 3500 ms, TE = 17/84 ms, FOV = 208 × 256 mm, matrix = 208 × 256, section thickness = 5 mm) and coronal turbo spin-echo T2-weighted images (TR = 4100 ms, TE = 143 ms, FOV = 324 × 384 mm, matrix = 324 × 384, section thickness = 5 mm). Structural imaging was visually assessed by a senior neuroradiologist for the presence or absence of supratentorial and infratentorial abnormalities and signal-intensity changes in patients and TD subjects.
Image Processing
A VBM study was conducted to investigate the local differences in GM and WM volume among ASD with ID, TD, and ID groups. The T1-weighted volumetric images were analyzed with the Statistical Parametric Mapping 8 (SPM8) package (http://www.fil.ion.ucl.ac.uk/spm/software/spm12/) and VBM8 toolbox (http://dbm.neuro.uni-jena.de/vbm8/). We used the Diffeomorphic Anatomical Registration by using the Exponentiated Lie algebra (DARTEL) algorithm,20 implemented in VBM8 to generate a study-specific template and to perform the registration of the subjects to that template.21
The VBM preprocessing steps are as follows: 1) checking for scanner artifacts and gross anatomic abnormalities in each subject; 2) image origin setting to the anterior commissure; 3) VBM8 segmentation of brain tissues with default settings based on the DARTEL template in Montreal Neurological Institute space derived from the IXI data base (http://www.brain-development.org); 4) creation of a study-specific template from all the segmented images by using the VBM8 toolbox; 5) a second VBM8 segmentation of brain tissues, with alignment of the subjects to the study-specific template and “nonlinear only” modulation to account for different individual brain sizes; 6) check for suboptimal overlapping by visual inspection of the normalized images and dishomogeneities by using a covariance matrix; and 7) spatial smoothing of the preprocessed GM and WM images, applying different isotropic Gaussian kernels such as 2-, 4-, 8-mm full width at half maximum kernels.
The GM, WM, and CSF volumes were calculated by the relevant VBM8 function, and the total intracranial volume, as the sum.
Statistical Analysis
Differences in age, total intracranial volume, total GM, total WM, and CSF volumes among the 3 groups were assessed by 2-way ANOVA by using group (ASD with ID, ID, and TD) and sex as independent variables. One-way ANOVA was performed to assess IQ differences.
Classic Parametric Analysis
A parametric statistical analysis based on the random field theory was performed. Two 1-way ANOVAs were designed by using the general linear model to investigate local GM and local WM volume differences among the 3 groups. We used age, sex, IQ, and total GM/WM volume as covariates in the local GM/WM volume analysis to correct for any differences due to nuisance variables. T tests were performed to investigate group differences in local GM/WM volume between the following: 1) ASD with ID and TD, 2) ASD with ID and ID, 3) ID and TD.
The above-described classic analysis was performed on normalized and modulated GM/WM images with the standard smoothing level (8-mm full width at half maximum) and, in addition, with lower smoothing levels (4-mm, 2-mm full-width half-maximum) to allow comparison with the following nonparametric approach.
Threshold-Free Cluster Enhancement Analysis
A TFCE nonparametric permutative statistical analysis was performed. The TFCE method is extensively described in the related article10 and implemented in the freely available toolbox (http://dbm.neuro.uni-jena.de/tfce/). Briefly, the TFCE algorithm takes an input raw statistical image and produces an output image in which the voxelwise values represent the amount of clusterlike local spatial support. Then, for each contrast, the group labels are randomly permuted to obtain an empirically derived null distribution against which one can compare the observed effects.
In the same way as in the classic parametric analysis, two 1-way ANOVAs with 4 nuisance regressors (age, sex, IQ, and total GM/WM volume) were designed by using the general linear model to investigate local GM and WM volume differences. Then, TFCE nonparametric tests were performed to investigate group differences in local GM/WM volume between the following: 1) ASD with ID and TD, 2) ASD with ID and ID, and 3) ID and TD. The group labels were randomly permuted 5000 times (default setting) for each test. A Bonferroni correction was applied to each pair-wise test in order to correct for multiple comparisons across groups.
TFCE does not explicitly require image smoothing and allows small smoothing kernels.10 Thus, the above-described TFCE analysis was performed on normalized and modulated GM/WM images with no smoothing and, in addition, with 2- and 4-mm full width at half maximum isotropic Gaussian kernels.
Results
The 3 groups were matched for age (F = 0.16, P = .985), and the 2 clinical groups were comparable for IQ. The mean IQ was 56 ± 7 (range, 41–66) and 53 ± 10 (range, 37–66) for ASD with ID and ID respectively (t = −1.127, P = .266). The mean IQ in TD was 103 ± 9 (range, 91–126), which is significantly higher than that in the 2 clinical groups (F = 251.41, P = .000).
Increased Total Volumes in ASD with ID
The 2-way unrelated ANOVA showed that a significant effect was obtained on total GM volume, total WM volume, and total intracranial volume for sex but not for group and their interactions. Males had total volume measures higher than females in all 3 groups. The main effect of group on CSF volume is significant, with significantly less volume in those with ID compared with TD (P = .016). Total volumes in the ASD with ID, ID, and TD groups and 2-way unrelated ANOVA output are shown in the Table.
Total volumes in ASD with ID, ID, and TD groups and 2-way unrelated ANOVA output
| ASD with ID (Mean) (SD) |
ID (Mean) (SD) |
TD (Mean) (SD) |
2-Way ANOVA (Mean) | |||||
|---|---|---|---|---|---|---|---|---|
| Male | Female | Male | Female | Male | Female | |||
| TIV (mL) | 1396.1 (116.2) | 1222.2 (61.7) | 1293.5 (136.2) | 1177.7 (105.7) | 1326.7 (100.1) | 1235.5 (74.7) | Group | 1.910 ± .156 |
| Sex | 16.793 ± .000 | |||||||
| Group × sex | .523 ± .595 | |||||||
| Total GM (mL) | 724.0 (92.4) | 569.6 (55,1) | 662.6 (71,5) | 628.2 (83,4) | 654.3 (40.8) | 602.5 (73.4) | Group | .377 ± .687 |
| Sex | 2.373 ± .000 | |||||||
| Group × sex | 14.003 ± .101 | |||||||
| Total WM (mL) | 411.8 (54.4) | 353.7 (31.8) | 378.4 (53.1) | 330.7 (47.3) | 387.5 (40.9) | 361.3 (31.7) | Group | 1.668 ± .196 |
| Sex | 10.874 ± .002 | |||||||
| Group × sex | .532 ± .590 | |||||||
| CSF (mL) | 260.2 (53.6) | 298.9 (56.7) | 252.5 (55.7) | 218.8 (51.3) | 284.8 (55.9) | 271.7 (51.4) | Group | 4.449 ± .015 |
| Sex | .032 ± .858 | |||||||
| Group × sex | 1.639 ± .202 | |||||||
Note:—TIV indicates total intracranial volume.
Classic Parametric VBM Analysis
No differences in local GM volumes and local WM volumes among the 3 groups survived the multiple comparison correction (P < .05 family-wise error–corrected), regardless of the smoothing kernel applied during preprocessing.
TFCE-Based VBM Analysis
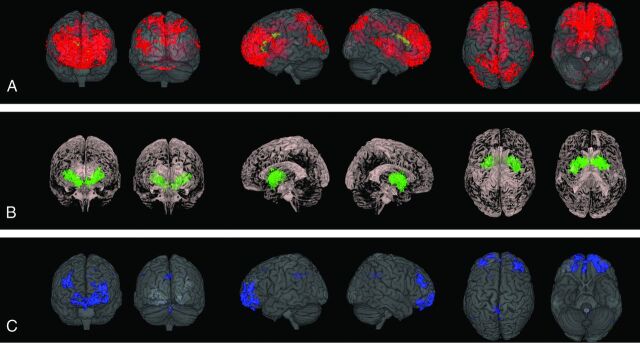
The TFCE-based analysis was sensitive to local GM and WM volume differences (P < .05 family-wise error–corrected), and Bonferroni-corrected P values were lower than the significant threshold (.05), regardless of the smoothing kernel applied during preprocessing. The effect of the smoothing was an enlargement of the blobs. TFCE nonparametric analysis results based on unsmoothed images are shown in Figure.
Fig 1.
TFCE nonparametric analysis (P < .05 family-wise error–corrected) based on unsmoothed images. A, ASD with ID shows cortical and subcortical GM decrease (red) and WM decrease (yellow) in comparison with TD. B, ID shows subcortical GM decrease (green) in comparison with TD. C, ASD with ID shows cortical GM decrease (blue) in comparison with ID.
Widespread Cortical and Subcortical Anomalies in ASD with ID
ASD with ID showed widespread cortical and subcortical GM decreases, compared with TD (Fig 1A, red). Frontobasal regions together with the anterior cingulate cortex and dorsolateral prefrontal cortex compounded the largest and most statistically significant cluster. More spotty differences were detected in the middle and posterior cingulate; parietal (precuneus and superior parietal lobule bilaterally, left inferior parietal lobule), occipital (cuneus bilaterally, left lateral occipital cortex, and right superior occipital gyrus), and temporal (right superior temporal gyrus and left middle temporal gyrus) cortices; insula; and thalamus.
Analysis focused on WM revealed decreased WM in the body, genu, and forceps minor of the corpus callosum (CC) (Fig 1A, yellow). Increased WM was detected in a small area in the midbrain.
Subcortical Anomalies in ID
GM decrease in ID compared with TD was detected in the basal ganglia and basal forebrain (Fig 1B, green). Two small areas of increased WM were found in the midbrain and the medulla oblongata.
Frontocingulate-Parietal Differences between ASD with ID and ID
GM decrease in ASD with ID compared with ID was detected in the fronto-orbital cortex, middle and posterior cingulate cortices, and precuneus (Fig 1C, blue).
Discussion
Classic VBM voxel-based inference and cluster-based inference are known to carry some criticisms such as smoothing kernel extent and statistical threshold.22,23 In theory, the definition should be based on the expected study-specific effect (that is actually unknown), while in common practice, the extent of the smoothing kernel and the statistical significance threshold are defined arbitrarily. TFCE was proposed to address the criticisms of smoothing kernel extent, threshold dependence, and localization of the results and to optimize the detection of both diffuse, low-amplitude signals and sharp, focal signals, while keeping strong control over family-wise error.10 Moreover, while cluster-based inference requires an adjustment to ensure homogeneity of the local smoothness, TFCE inference is robust to nonstationarity.24 Several recent studies transversely adopted TFCE in several neuroimaging modalities, and it was demonstrated that it improves the sensitivity of VBM statistical analysis in neurodegenerative and psychiatric diseases.8–10
Our goal was to detect structural anomalies in the under-researched condition of ASD with ID, also directly comparing it with nonsyndromic ID, and to verify the improvement of structural anomaly detection in neurodevelopmental disorders by using TFCE. In this study, no significant voxelwise differences among the groups survived correction for multiple comparisons by using a classic parametric VBM approach, and significant voxelwise differences among the groups survived correction for multiple comparisons by using a TFCE nonparametric statistical approach. The cortical and subcortical alterations in ASD with ID are discussed in the following paragraphs.
Widespread Focal Cortical Alterations in ASD with ID
Local GM volume decrease was distributed throughout the lobes in ASD with ID. The larger the smoothing kernel applied during image preprocessing, the larger were the blobs. When no smoothing was applied, anomalies were characterized by a focal appearance, the attenuation of which seemed not constant across the cortical lobes. Indeed, such attenuation seemed to follow a negative gradient from the ventromedial prefrontal cortex to the occipital and temporal regions, through the cingulate and medial parietal cortex. Image smoothing did impact on anomaly focality and made it difficult to observe the variation of focal abnormality attenuation across the lobes. Thus, we showed the results based on unsmoothed images. However, we used the analysis based on smoothed images to exclude results based on the unsmoothed images being due to noise and to make classic and TFCE-based VBM analyses comparable (ie, the same preprocessing pipeline). The TFCE method allows the use of unsmoothed images that in turn, allows investigating focal cortical anomalies.
Among the cortical regions altered in ASD with ID, significant differences in the frontocingulate-parietal cortex were also detected between ASD with ID and ID, while ID had no significant anomalies in cortical regions. The frontocingulate-parietal network involves cortical areas that some authors have assumed to underlie general intelligence,25 while others are associated with specific higher cognitive abilities, such as working memory or executive functions.26 The present study suggests that the frontocingulate-parietal cortex may be the eligible key region for further investigations aiming at detecting imaging biomarkers in ASD with ID.
The widespread focal cortical anomalies in ASD with ID shown in the present study might be related to patches of disorganization in the neocortex27; subtle focal cortical dysplasias are most abundant within the prefrontal lobes and are explicitly associated with focal and distributed thinning of the cortex in postmortem investigations.28
Corpus Callosum Alterations in ASD with ID
The genu and body of the CC showed decreased local WM volume in ASD with ID. The CC is one of the most consistently altered regions in ASD, according to conventional MR imaging29 and DTI.30 The anterior sector of the CC seems to be more involved than the posterior sectors.31 WM decrease in the genu and body of the CC supports the hypothesis of impaired interhemispheric communication, particularly involving the frontal and parietal regions.
No significant differences were detected in the CC by comparing ASD with ID and ID as well as ID and TD. Diffusion MR imaging studies are needed to subcharacterize the WM in ASD with ID.
Basal Ganglia Alterations Shared between ASD with ID and ID
Alterations of the basal ganglia were repeatedly described in patients with ASD, and they were often found to correlate with impaired motor performance or repetitive and stereotyped behavior.32,33 Alterations in the basal forebrain were also previously described in patients with ASD with ID.6 The basal forebrain comprises a group of structures located in the medial and ventral surface of the frontal lobe, implicated in a number of cognitive functions and social behavior patterns.34
In the present study, local volumetric anomalies in the basal ganglia and basal forebrain were detected in both ASD with ID and ID. Further multimodal research in the complex basal ganglia system is needed to deepen the role of basal ganglia alteration in neurodevelopmental disorders.
Present Limitations and Future Prospects
One limitation of the present study may be the restricted number of participants (75 children in total). Twenty-five participants in each group were the largest sample available, taking into account image artifacts, exclusion criteria, IQ matching between the 2 clinical groups, and age matching among the 3 groups. Larger samples would also allow studying the dynamics of the structural anomalies across ages. Although our experimental model controlled for sex differences and related total volume differences, the study would have benefited from sex matching and a balanced sex ratio.
In addition, the comparison with a group with ASD and normal intelligence would deepen the comprehension of the structural anomalies in ASD with ID.
The administration of propofol to only TD children younger than 6 years of age might impact the results. To exclude this outcome, we performed quantitative image quality control by the relevant tool available in VBM8, besides a qualitative visual check of original and preprocessed images. The images of the unsedated TD subjects did not differ from those of subjects who were administered propofol.
Future neuroimaging research ought to focus on studying the different features that characterize the cortical surfaces (ie, intensity, cortical thickness, surface area, sulcal depth, gyrification) in ASD with ID, taking into account the focal nature of the expected anomalies. In light of the recent postmortem studies on the laminar architecture in ASD,27,28 the investigation into each of the 6 cortical layers would be crucial. However, in vivo detection of a particular cortical layer is still a challenge for MR brain imaging and future developments might allow ultra-high-resolution acquisitions in clinical settings.
Conclusions
The present voxelwise structural MR imaging investigation shows widespread focal cortical anomalies and subcortical alterations in ASD with ID. The comparison with nonsyndromic ID suggests the frontocingulate-parietal cortex as the key region eligible for further investigations to detect imaging biomarkers in ASD with ID. The detection of structural alterations in neurodevelopmental disorders may be dramatically improved by the TFCE statistical approach.
ABBREVIATIONS:
- ASD
autism spectrum disorder
- CC
corpus callosum
- ID
intellectual disability
- IQ
intelligence quotient
- TD
typically developing
- TFCE
threshold-free cluster enhancement
- VBM
voxel-based morphometry
References
- 1. American Psychiatric Association; DSM-5 Task Force. Diagnostic and Statistical Manual of Mental Disorders: DSM-5. Arlington, Virginia: American Psychiatric Association; 2013 [Google Scholar]
- 2. Autism and Developmental Disabilities Monitoring Network Surveillance Year 2010 Principal Investigators. Prevalence of autism spectrum disorder among children aged 8 years-autism and developmental disabilities monitoring network, 11 sites, United States, 2010. Surveillance Summaries. March 28, 2014. http://www.cdc.gov/mmwr/preview/mmwrhtml/ss6302a1. Accessed October 14, 2015. [PubMed]
- 3. Lai MC, Lombardo MV, Baron-Cohen S. Autism. Lancet 2014;383:896–910 10.1016/S0140-6736(13)61539-1 [DOI] [PubMed] [Google Scholar]
- 4. Boddaert N, Chabane N, Gervais H, et al. Superior temporal sulcus: anatomical abnormalities in childhood autism: a voxel based morphometry MRI study. Neuroimage 2004;23:364–69 [DOI] [PubMed] [Google Scholar]
- 5. Bonilha L, Cendes F, Rorden C, et al. Gray and white matter imbalance: typical structural abnormality underlying classic autism? Brain Dev 2008;30:396–401 10.1016/j.braindev.2007.11.006 [DOI] [PubMed] [Google Scholar]
- 6. Riva D, Bulgheroni S, Aquino D, et al. Basal forebrain involvement in low-functioning autistic children: a voxel-based morphometry study. AJNR Am J Neuroradiol 2011;32:1430–35 10.3174/ajnr.A2527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Riva D, Annunziata S, Contarino V, et al. Gray matter reduction in the vermis and CRUS-II is associated with social and interaction deficits in low-functioning children with autistic spectrum disorders: a VBM-DARTEL study. Cerebellum 2013;12:676–85 10.1007/s12311-013-0469-8 [DOI] [PubMed] [Google Scholar]
- 8. Rajagopalan V, Yue GH, Pioro EP. Do preprocessing algorithms and statistical models influence voxel-based morphometry (VBM) results in amyotrophic lateral sclerosis patients? A systematic comparison of popular VBM analytical methods. J Magn Reson Imaging 2014;40:662–67 10.1002/jmri.24415 [DOI] [PubMed] [Google Scholar]
- 9. Radua J, Canales-Rodríguez EJ, Pomarol-Clotet E, et al. Validity of modulation and optimal settings for advanced voxel-based morphometry. Neuroimage 2014;86:81–90 10.1016/j.neuroimage.2013.07.084 [DOI] [PubMed] [Google Scholar]
- 10. Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage 2009;44:83–98 10.1016/j.neuroimage.2008.03.061 [DOI] [PubMed] [Google Scholar]
- 11. Lord C, Risi S, Lambrecht L, et al.. The Autism Diagnostic Observation Schedule-Generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord 2000;30:205–23 [PubMed] [Google Scholar]
- 12. Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord 1994;24:659–85 [DOI] [PubMed] [Google Scholar]
- 13. Wechsler D. WISC-III: Wechsler Intelligence Scale for Children. Italian Adaptation by Orsini and Picone. Firenze: Organizzazioni Speciali; 2006 [Google Scholar]
- 14. Wechsler D. Wechsler Preschool and Primary Scale of Intelligence-III: Italian Adaptation by Sannio Fancello G. and Cianchetti C. Firenze: Organizzazioni Speciali; 2008 [Google Scholar]
- 15. Griffiths R. GMDS-ER Griffiths Mental Development Scales-Extended Revised. Firenze: Giunti Organizzazioni Speciali; 2006 [Google Scholar]
- 16. Griffiths R. The Griffiths Mental Development Scales 1996 Revision. Henley: Association for Research in Infant and Child Development; 1996 [Google Scholar]
- 17. Sutcliffe AG, Soo A, Barnes J. Predictive value of developmental testing in the second year for cognitive development at five years of age. Pediatr Rep 2010;2:e15 10.4081/pr.2010.e15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rutter M, Bailey A, Lord C. The Social Communication Questionnaire. Los Angeles: Western Psychological Services; 2003 [Google Scholar]
- 19. Erbetta A, Bulgheroni S, Contarino V, et al. Neuroimaging findings in 41 low-functioning children with autism spectrum disorder: a single-center experience. J Child Neurol 2014;29:1626–31 10.1177/088307381351185631 [DOI] [PubMed] [Google Scholar]
- 20. Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage 2007;38:95–113 [DOI] [PubMed] [Google Scholar]
- 21. Yassa MA, Stark CE. A quantitative evaluation of cross-participant registration techniques for MRI studies of the medial temporal lobe. Neuroimage 2009;44:319–27 10.1016/j.neuroimage.2008.09.016 [DOI] [PubMed] [Google Scholar]
- 22. Henley SM, Ridgway GR, Scahill RI, et al. ; EHDN Imaging Working Group. Pitfalls in the use of voxel-based morphometry as a biomarker: examples from Huntington disease. AJNR Am J Neuroradiol 2010;31:711–19 10.3174/ajnr.A1939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shen S, Sterr A. Is DARTEL-based voxel-based morphometry affected by width of smoothing kernel and group size? A study using simulated atrophy. J Magn Reson Imaging 2013;37:1468–75 10.1002/jmri.23927 [DOI] [PubMed] [Google Scholar]
- 24. Salimi-Khorshidi G, Smith SM, Nichols TE. Adjusting the effect of nonstationarity in cluster-based and TFCE inference. Neuroimage 2011;54:2006–19 10.1016/j.neuroimage.2010.09.088 [DOI] [PubMed] [Google Scholar]
- 25. Jung RE, Haier RJ. The Parieto-Frontal Integration Theory (P-FIT) of intelligence: converging neuroimaging evidence. Behav Brain Sci 2007;30:135–54; discussion 154–87 [DOI] [PubMed] [Google Scholar]
- 26. Blair C. How similar are fluid cognition and general intelligence? A developmental neuroscience perspective on fluid cognition as an aspect of human cognitive ability. Behav Brain Sci 2006;29:109–25; discussion 125–60 [DOI] [PubMed] [Google Scholar]
- 27. Stoner R, Chow ML, Boyle MP, et al. Patches of disorganization in the neocortex of children with autism. N Engl J Med 2014;370:1209–19 10.1056/NEJMoa1307491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Casanova MF, El-Baz AS, Kamat SS, et al. Focal cortical dysplasias in autism spectrum disorders. Acta Neuropathol Commun 2013;1:67 10.1186/2051-5960-1-67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Stanfield AC, McIntosh AM, Spencer MD, et al. Towards a neuroanatomy of autism: a systematic review and meta-analysis of structural magnetic resonance imaging studies. Eur Psychiatry 2008;23:289–99 [DOI] [PubMed] [Google Scholar]
- 30. Travers BG, Adluru N, Ennis C, et al. Diffusion tensor imaging in autism spectrum disorder: a review. Autism Res 2012;5:289–313 10.1002/aur.1243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bellani M, Calderoni S, Muratori F, et al. Brain anatomy of autism spectrum disorders, I: focus on corpus callosum. Epidemiol Psychiatr Sci 2013;22:217–21 10.1017/S2045796013000139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Estes A, Shaw DW, Sparks BF, et al. Basal ganglia morphometry and repetitive behavior in young children with autism spectrum disorder. Autism Res 2011;4:212–20 10.1002/aur.193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hardan AY, Kilpatrick M, Keshavan MS, et al. Motor performance and anatomic magnetic resonance imaging (MRI) of the basal ganglia in autism. J Child Neurol 2003;18:317–24 [DOI] [PubMed] [Google Scholar]
- 34. Baxter MG, Chiba AA. Cognitive functions of the basal forebrain. Curr Opin Neurobiol 1999;9:178–83 [DOI] [PubMed] [Google Scholar]